Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Skin cleansers generally come in one of two types: soap-based and synthetic detergents, or syndets. While the latter can effectively maintain the native skin structure, function and integrity, the former tends to negatively affect the skin by causing barrier disruption, lipid dissolution and pH alteration. Despite this, soap is still often preferred, possibly due to the negative connotations around anything that is not perceived as ‘natural’.
- capacity
- charge
- ingredient
- mildness
- pH
- cleanser
- skin
- soap
- surfactant
- syndet
1. Introduction
The stratum corneum (SC) is the uppermost layer of the skin’s epidermis, and is a biochemically complex but highly organised interface with the external environment. The SC is usually described as a ‘bricks and mortar’ structure in which the protein enriched, flattened corneocytes (dead keratinocytes lacking vital cellular organelles) are the ‘bricks’, and the lipid-rich matrix in which they are embedded is the ‘mortar’ [1]. This lipid matrix predominantly contains ceramides (40–50%), cholesterol (25%) and free fatty acids (10–15%) [2][3][4][5]. These three major classes of lipids within the SC are biophysically and biochemically distinct from other conventional eukaryotic membrane constituents (e.g., glycerolipids, sphingolipids, sterols) that are involved in the structural and functional landscape of the cell membrane’s lipid bilayers [6]. SC lipid precursors are generated during keratinocyte differentiation, and are afterwards intracellularly packaged into lamellar bodies in a highly ordered manner and subsequently enzymatically converted and eventually secreted into the extracellular domain of the SC [2][3][7]. The SC lipids spontaneously and in an orderly manner form multiple bilayers, which interact with corneocyte envelope-bound lipids [4][8]. In deeper layers of the SC, lipids are predominantly ordered into a densely packed orthorhombic crystalline configuration, which acts as a restrictive barrier to liquid transport. Closer to the SC surface, the lipids form a dispersed hexagonal lattice configuration that permits more liquid to pass through more freely [9][10].
The highly ordered SC lipid configuration is characterised by its unique properties, composition, and the specialised compartmentalisation within the lamellar lipid membrane, which maintains SC water content, regulates water flux, and modifies the rate and magnitude of transepidermal water loss (TEWL). Such mechanisms are highly dynamic and function continuously to maintain homeostasis of the SC, the epidermis and the skin in its entirety [2]. As such, the SC is a robust factory that is in operation at all times, ensuring the maintenance of the skin’s adaptive and protective barrier [2][11]. Exogenous stressors can impede the skin’s protective properties, so reducing exposure to, and minimising practices such as skin cleansing with, harsh cleansers (e.g., alkaline soaps) that can cause perturbation and damage to the SC proteins, lipids and natural moisturising factor (NMF) components (e.g., free amino acids, sugars) is critical to decreasing the stress load on the skin’s structural and functional integrity [2].
It is generally recognised [7][9][12][13][14] that all types of skin, from healthy to diseased, infant to aged, need to be kept clean in order to preserve their barrier properties. The main objective of skin cleansing is to remove impurities from the skin’s surface [15][16] and to control its odour, without removing protective SC surface proteins and lipids, affecting skin microbiota [17] or altering pH [13][18]. This can be a substantial challenge, as the skin’s composition and barrier integrity are intricate, inconsistent, and dynamic, as described above [13][18].
As most of the impurities and contaminants that are found on the skin’s surface are not water-soluble, cleansing the skin with water alone is inadequate; hence, there is a need for surfactant-containing products [15]. Unlike water, surfactant-containing products are capable of breaking down and emulsifying most of those skin impurities and contaminants into finer particles and enabling their subsequent detachment and removal from the surface of the skin [3][7]. This makes surfactants a key component of skin maintenance. However, not all surfactants are created equal, with some, namely soap-based surfactants, creating more problems than they address [3][9][10][13][16][17][19][20][21][22][23][24][25][26][27][28][29][30][31].
2. Soaps vs. Syndets: Similarities and Differences
A diverse range of skin cleansers exist today, but they all generally fall into two types: soap-based and syndets. Both soap- and syndet-based cleansers contain at least one (often more than one) surfactant, a class of organic compounds that are amphiphilic/amphipathic, that is, they contain both nonpolar or hydrophobic (water-hating and lipid-loving) moieties, also known as ‘tails’, and polar or hydrophilic (water-loving) moieties, also known as ‘heads’. Therefore, they are soluble in both water and organic solvents [21]. Due to surfactants’ unique chemistry and characteristics, hydrophobic compounds present in excess sebum and other undesired substances (e.g., dirt, oils) on the skin’s surface are washed away with greater ease than could be achieved with water alone [7][9][12][13].
While soaps and syndets are similar in that they cleanse dirt and impurities from the surface of the skin, their distinct chemical properties and physiological effects can be markedly different [3][9][10][13][16][17][19][20][21][22][23][24][25][26][27][28][29][30][31] (Figure 1).
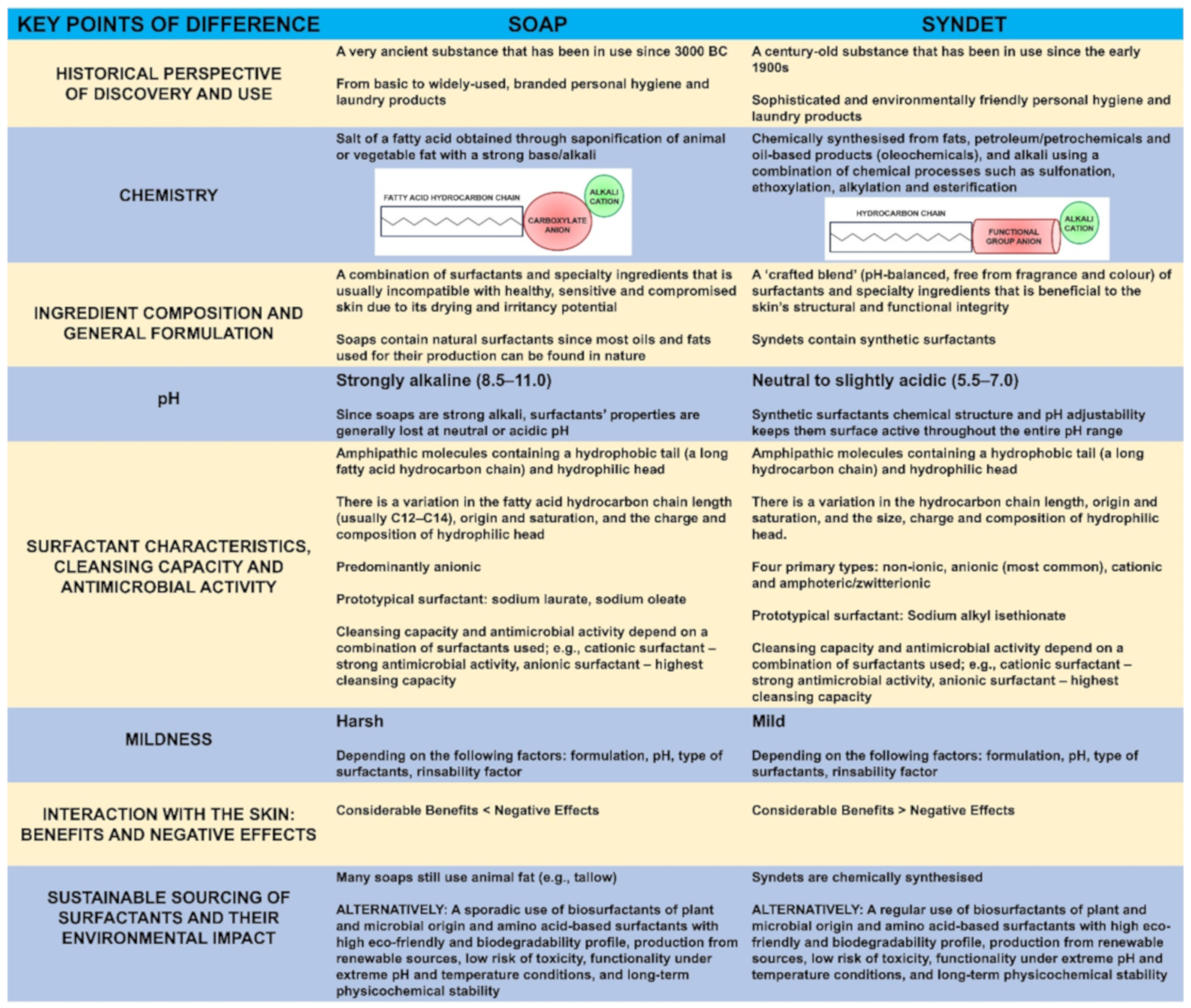
2.1. Soaps vs. Syndets: Key Points of Difference
The key points of difference between soaps and syndets (Figure 1) include the following: their historical perspective of discovery and use [19][29]; chemistry [9][16][24][29]; ingredient composition and general formulation [3][17][25]; pH [22][24][25]; surfactant characteristics [13][21][32], cleansing capacity [13][16][21][27][32][33] and antimicrobial activity [23][28]; mildness [13][16][21][27]; sustainable sourcing of surfactants and their environmental impact [26][30][34][35][36]; and interaction with the skin [3], associated benefits and negative effects [3][9][10][20][25].
3. Effects of Mild Syndets and Harsh Soaps on the Skin
Selecting an optimal and suitable cleanser is key to maintaining the skin acid mantle, its barrier function and integrity, and preserving the skin’s overall health at the same time. The exact needs of the skin differ with age and skin condition (Figure 2). Therefore, it is essential to understand these needs and differences in order to identify the most suitable cleanser for every situation. For instance, thinner skin can be more prone to TEWL, so soaps may increase this negative effect by exacerbating TEWL, whereas syndets may limit further TEWL and improve hydration. It seems that the interaction of mild syndets with the skin can be beneficial for almost every situation and will tip the balance as the positives outweigh the negatives, whereas the interaction of harsh soaps with the skin can have a much greater potential to cause a range of negative effects such as impaired barrier function, dryness and irritancy, and will tip the balance towards the negative effects (Figure 2). Therefore, fine-tuning of the complex characteristics of cleansers is absolutely necessary in order to produce cleansing formulations with optimal benefits and minimal adverse effects [7][9][12][13].
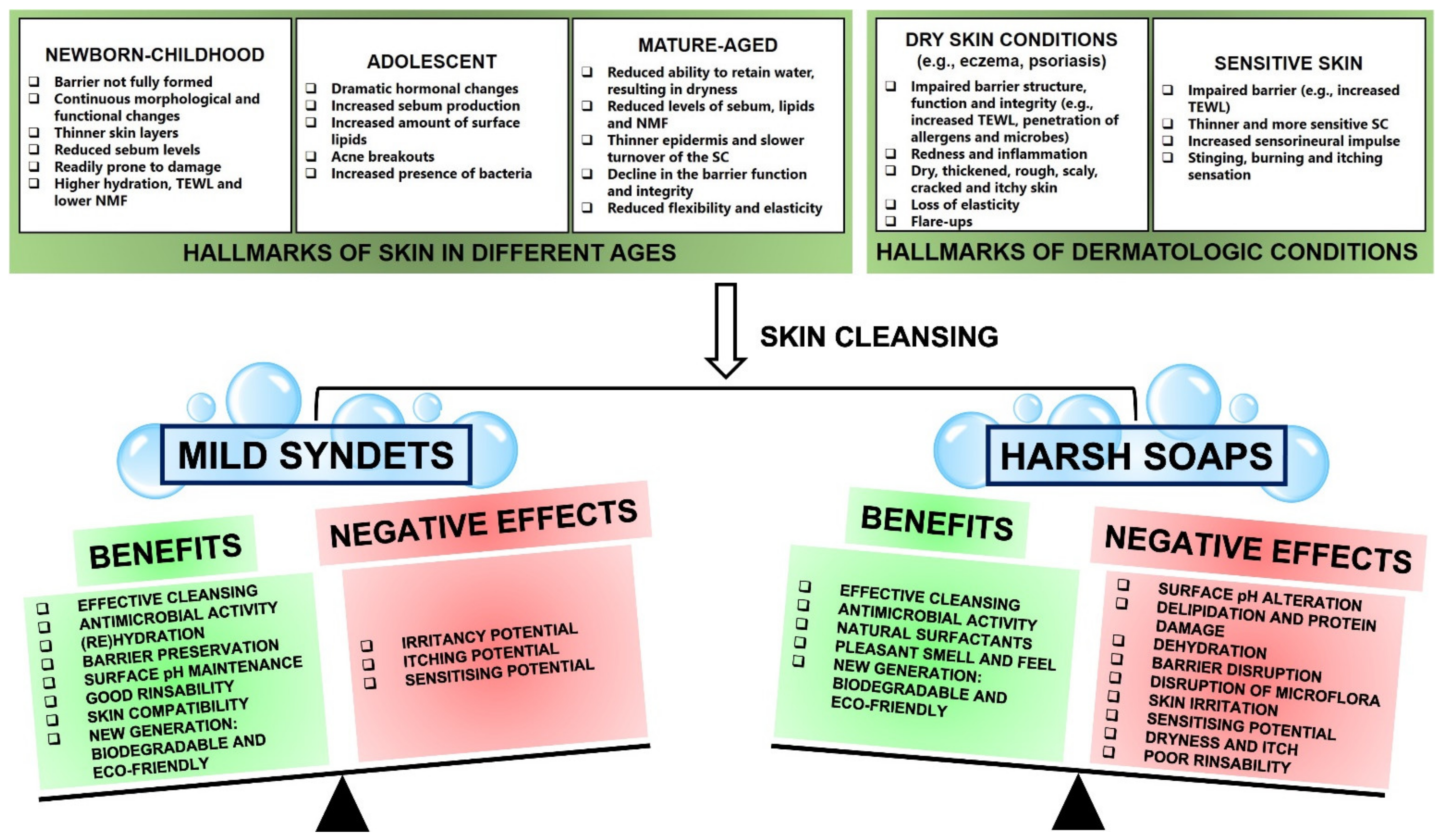
3.1. Mild Syndets: Considerable Benefits and Minimal Negative Effects
Children in general, especially newborns and infants, have a unique skin structure and physiology. While newborn skin is still not fully formed, it is sufficiently mature to cope with the usual demands of life. The skin continuously undergoes a period of rapid anatomical and physiological transformation, particularly in relation to SC hydration, TEWL, sebum and NMF production, development of the skin barrier (in terms of structural and functional integrity), the transition to a more acidic skin surface and skin maturation [13][38][39]. This process of maturation and morphological and functional changes continues for several years, indicating that newborn-childhood skin is particularly vulnerable to sensitisation, inflammation, irritation and unfavourable changes in skin barrier function [13][39] (Figure 2). Therefore, it is an imperative to use a suitable cleanser with mild properties for a child’s skin [39].
At puberty, the levels of circulating growth and reproduction hormones increase dramatically, causing various changes in the skin, including a high prevalence of acne [13]. A defining characteristic of acne is abnormal sebum production, making the skin oily, an issue that can be compounded by the drying nature of acne medication, such as benzoyl peroxide. Thus, effective cleansers, especially facial cleansers for acne management, must satisfy two opposing needs: (1) removal of sebum and (2) maintaining skin moisture [13]. A mild, fragrance-free and irritant-free cleanser with good rinsability is the recommended cleanser for acne management [7][13]. The cleansing regimen should suit the needs of the individual patient [7][14] (Figure 2).
At the other end of the spectrum, aged skin is characterised by changes such as impaired barrier function, thinner epidermis, reduced skin elasticity and decreased sweat and sebum production. Such changes ultimately result in a reduced ability of the skin to retain water, eventually leading to dry and fragile skin [13][40]. When it comes to cleansing aged skin, the recommendations remain the same as that for young skin: avoid alkaline cleansers and use products that are mild in nature, and able to maintain or even replenish the skin’s moisture [40] (Figure 2).
In individuals with dry skin conditions, including eczema, the use of traditional soap with its characteristic high pH can aggravate the skin, leading to loss of intracellular lipids, leaving the skin with a red, rough and scaly appearance [13][41]. This damage can potentially expose dermal nerve endings (a hallmark of sensitive skin), resulting in itching, burning, and pain [42]. Skin barrier impairment can also contribute to the penetration of allergens and an increased colonisation of bacteria such as S. aureus. Again, when it comes to cleansing, the recommendations remain similar: use mildly acidified syndets for cleansing, with an adjusted pH value in order to reduce the participation of infectious organisms, irritants, or allergens as well as to minimise irritation and itching potential [7][13][14][41] (Figure 2).
Unfortunately, even syndets with favourable mildness can potentially remove skin’s essential constituents, compromise the integrity and functionality of the SC, and can inevitably result in some weakening of the skin barrier, sensitisation and irritation (Figure 2). Thus, skin cleansing activity should be conducted with care because the careless use of skin cleansers in general, and especially the use of harsh alkaline soaps, will undoubtedly cause adverse skin reactions [43]. A best-suited and mild-in-nature cleanser such as a syndet should effortlessly and simultaneously maintain a fine balance between skin cleansing on the one hand and the preservation of the skin’s homeostatic properties on the other, with minimal to no irritation, disruption of, or damage to the skin’s physiological parameters, including hydration, acid mantle, and thus, overall barrier function [3][7].
3.2. Harsh Soaps: Extensive Short-Term and Long-Term Negative Effects and Some Benefits
Harsh soaps have the potential and aggressiveness to initiate changes to SC proteins, lipids and pH, disrupt resident microflora, and progressively increase damage over time, which in turn can cause a fundamental destruction of the barrier integrity that underpins skin health (Figure 2). Overall, the use of harsh soaps can cause cumulative skin effects including dryness, roughness, scaling, after-wash tightness, irritation and itching [7][14][17][20][37] (Figure 3).
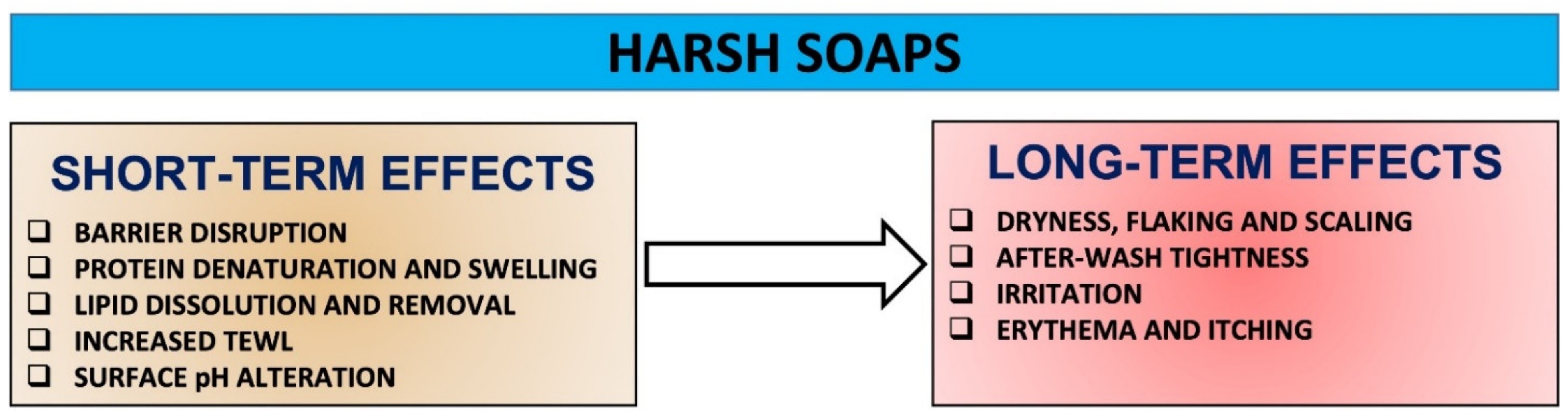
Most of the water absorbed by the SC during cleansing is present within the corneocytes. This results in a significant protein denaturation and swelling, and ultimate keratinocyte damage. Harsh soaps increase the swelling further, and the extent of surfactant-induced swelling is dependent upon the surfactant’s nature and its irritation potential. In addition, harsh soaps binding to proteins may also reduce the water-holding capacity of proteins. For example, the extent of swelling in the presence of sodium laurate-containing soap is significantly higher than that in the presence of a sodium cocoyl isethionate-containing syndet [20]. Furthermore, harsh soaps have been shown to remove NMF components and cause damage to the corneocyte envelope [20]. Therefore, the higher loss of water-soluble proteins after a single wash with soap vs. syndet is consistent with the greater damage potential of soap, depending on its structural and charge-density differences, direct effects of pH on the SC, and/or indirect effects of pH on the solution chemistry of charged head groups [7][14][20]. As water evaporates at a rapid rate from the upper layers of the skin, a differential stress is created in the SC, and this is thought to be the origin of the after-wash tightness [20].
Harsh soaps have an ability to disrupt and potentially damage bilayer lipids by extracting endogenous cholesterol, fatty acids and ceramides or intercalating into the lipid bilayer. Lipid barrier dissolution has numerous clinical consequences for the skin, including dryness, increased TEWL, fissuring, flaking, erythema and itch [14]. For example, insertion of anionic surfactants into the lipid bilayer can induce charge disruption in the bilayer and abruptly disturb membrane packing and organisation, permeability and overall structural and functional integrity. Even a subtle or partial removal of such lipids can make the lipid bilayer unstable [14][20]. It was shown that a harsh soap removes more cholesterol than a syndet [20]. While the exact reasons for this difference are not clear at present, it is likely that the alkaline pH of soap allows ionisation of the bilayer fatty acids, allowing easier cholesterol extraction from the SC. Furthermore, it is possible that the increased swelling of soap-damaged SC allows deeper layers of the SC to be readily exposed to the soap [20].
This entry is adapted from the peer-reviewed paper 10.3390/molecules27062010
This entry is offline, you can click here to edit this entry!
