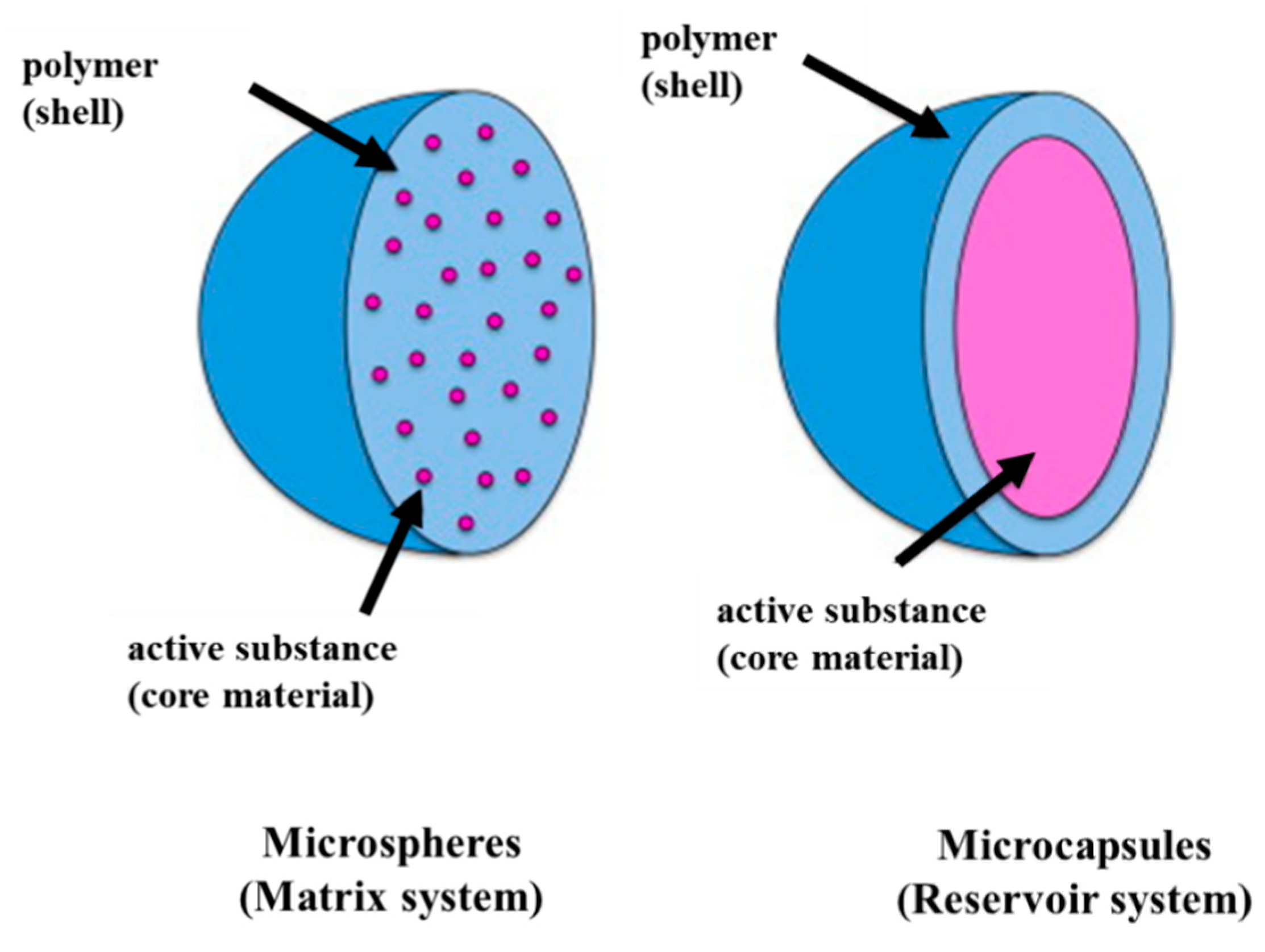
|
Microencapsulation Method
|
Wall Material
|
Antimicrobial Agent
|
Encapsulation Efficiency
|
Main Results
|
References
|
|
Inclusion
|
β-cyclodextrin
|
Eugenol
|
-
|
The eugenol-βCD complexes showed enhanced antibactieral activity compared to free eugenol (a concentration of eugenol higher than 5.03 mmol L−1 inhibited the growth of Staphylococcus aureus and Escherichia coli)
|
[11]
|
|
Inclusion (KN-FD)
|
HPBCD
|
Carvacrol
|
78.09% for KN, and 83.74% for FD
|
Encapsulated carvacrol showed a lower minimum concentration (300 μg/mL) than free carvacrol (1000 μg/mL) for both bacteria, Escherichia coli and Salmonella enterica
|
[12]
|
|
Inclusion (KN-FD)
|
β-cyclodextrin
|
Thymol and thyme oil
|
71 to 83%
|
β- cyclodextrine inclusion complexes were able to inhibit Escherichia coli at lower concentrations (0.37 mg/mL) than free oils.
|
[13]
|
|
Inclusion (by spray drying and precipitation methods)
|
HPBCD, β-CD
|
Lemongrass volatile oil
|
56–60% and 26–29% using β-CD and HP-β-CD, respectively
|
More effective inclusion of lemongrass oil with beta-CD
|
[14]
|
|
Inclusion
|
Β-cyclodextrin, HE-β-CD, HP-β-CD
|
Eugenol
|
-
|
The inclusion process was deduced to be an exothermic and enthalphy-driven process
|
[15]
|
|
Spray drying
|
Low methoxyl pectin
|
Lysozyme
|
-
|
Higher pectin concentrations (above 0.5 g/L) preserved lysozyme structure and activity
|
[16]
|
|
Spray drying
|
Gum arabic, starch, maltodextrin, inulin
|
Rosemary EO
|
-
|
The combination of modified starch and inulin was shown to be a viable substitute for gum arabic in foods
|
[17]
|
|
Spray drying
|
Modified starch, gum arabic, maltodextrin
|
Oregano EO
|
-
|
The inlet air temperature and the emulsion feed rate significantly affected the powder recovery, moisture content and the oil retention
|
[18]
|
|
Spray drying
|
Zein
|
Nisin
|
-
|
Encapsulated nisin was more effective than free antimicrobials in inhibiting the growth of L. monocytogenes
|
[19]
|
|
Simple coacervation
|
PVA crosslinked by glutaraldehyde
|
Lemongrass EO
|
-
|
When SDS at 0.03 wt.% was used, no agglomeration was observed
|
[20]
|
|
Simple coacervation
|
Gelatin microparticles crosslinked with glutaraldehyde
|
Holy basil EO (HBEO)
|
95.41%
|
Extended shelf-life of microencapsulated HBEO up to 18 months at 25 °C
|
[21]
|
|
Complex coacervation
|
Gelatin–gum arabic crosslinked with genipin
|
Mustard seed EO
|
-
|
Genipin-hardened microcapsules exhibited strong chemical stability with a particle size of mainly 5–10 μm
|
[22]
|
|
Complex coacervation
|
Soy protein–pectin
|
Propolis extract
|
72.01% and 66.12% for formulations with 2.5 and 5.0 g/100 mL of colloids, respectively
|
This process preserves the phenolic and flavonoids compounds with antioxidant activity and inhibitory activity of S. aureus
|
[23]
|
|
Complex coacervation
|
Whey protein isolate–chitosan
|
Garlic extract
|
51% to 61%
|
The CH/WPI complexes are revealed to be good alternatives for use as wall systems
|
[24]
|
|
Single emulsion diffusion method
|
Poly lactic acid (PLA)
|
Nisin
|
12 to 16%
|
The encapsulation efficiency was enhanced with the increase in nisin loading in the aqueous solution
|
[25]
|
|
Emulsion diffusion
|
Polycaprolacton (PCL)
|
Eugenol
|
100%, 90.9% and 89.1% for PCL, β-CD eugenol and 2-HP-β-CD eugenol, respectively
|
The emulsion–diffusion method was more effective for eugenol encapsulation to protect against light oxidation during storage time
|
[26]
|
|
s/o/w emulsion–evaporation extraction
|
Poly (lactic-co-glycolic) (PLGA)
|
Lysozyme
|
73%
|
More hydrophilic polymers were less prone to protein adsorption
|
[27]
|
|
Alginate microbeads
|
Calcium alginate–cellulose nanocrystals (CNC)
|
Nisin
|
-
|
The beads containing nisin significantly reduced the L. monocytogenes counts after 28 days of storage compared with free nisin
|
[28]
|
|
Alginate microbeads
|
Calcium–Alginate
|
Clove, thyme and cinnamon EOs
|
90 to 94%
|
Encapsulation in Ca-alginate microspheres could effectively reduce the evaporation rate of EOs
|
[29]
|
|
Alginate microbeads
|
Sodium alginate–guar gum
|
Nisin
|
36.35%
|
The encapsulation efficiency of nisin under optimal conditions was 36.65%
|
[30]
|
|
Ionic gelation
|
Sodium alginate
|
Propolis extract
|
99.3%
|
Na-alginate encapsulation increased the bioavailability of propolis extract
|
[31]
|
|
SAS
|
PLGA and CaCo3
|
Lysozyme
|
~60%
|
The supercritical CO2 process offers optimal conditions for protein stability and integrity and permitted the retention of 90% of the biological activity of lysozyme
|
[32]
|
|
Liposome
|
PC, PC/PG
|
Nisin
|
89–91, 78–83 and 72–78% for PC/PG 6:4, PC/PG 8:2 and PC, respectively
|
Liposomes formulated with PC and PG appeared to be relatively stable to pasteurization protocols
|
[33]
|
|
Liposome and emulsification method
|
Lecithin–chol
Alginate, chitosan or starch
|
Plant herbs, spices and lyzozyme
|
-
|
Particles with co-encapsulated herbs and lysozyme are more active against different types of bacteria
|
[34]
|
|
Vibrating technology
|
alginate
|
Nisin
|
75%
|
Microcapsules efficiently protected nisin from protease activity and retarded nisin release
|
[35]
|
3. Microencapsulation of Natural Food Antimicrobials
Applying GRAS natural antimicrobials in a food matrix can lead to healthier and safe products for consumers
[36], but the range of antibacterial activity of these compounds against different microorganisms as well as their compatibility and interactions with produced microcapsules and ability of microcapsules to protect entrapped agents and release core material at the desired rate should be evaluated. Antimicrobial activity of different agents can be increased or decreased via microencapsulation, unlike free agents, which depend on the composition of the antimicrobial agent and encapsulation method used.
3.1. Essential Oils
Limitations in the direct addition of EOs in food products include: alteration in sensory properties due to their very low flavor threshold, being hydrophobic and highly insoluble in water, resulting in low antibacterial activity in moist foods, and being volatile and chemically unstable because of oxidation, volatilization and chemical interactions. The antibacterial activity of EOs is determined through evaluating the minimum inhibitory concentration (MIC) in many studies. In brief, high amounts of EOs should be added directly to food to exhibit the same antimicrobial activity rather than antibacterial assays in vitro because of the probable effectiveness of high amounts of some food components such as fats and proteins in preserving bacterial strains against EO antibacterial activity in some way. The bacteria present in the aqueous phase of food is mostly out of reach of EO, which is dissolved in the lipid phase. Additionally, antimicrobial activity against targeted bacteria can be restricted by low diffusion of bioactive agents in food products with low water activity
[37]. Considering demands for effective application of natural antimicrobials such as EOs in food industries, microencapsulation of these compounds can be used as an alternative technique to solve problems relating to their direct addition
[11][38][39][40].
Controlled release of antimicrobial agents from the films without any changes in their antimicrobial capacity could be achieved via microencapsulation rather than direct addition of these agents into the film matrix. The release of encapsulated thymol and carvacrol (1%, 2%, 5% and 10% each) by the emulsification method (O/W) from bi-axially-oriented polypropylene (BOPP) films was studied over a course of 28 days at 4 °C
[41]. EOs were more effective against yeast while the highest MIC (375 ppm) was obtained for carvacrol against
Escherichia coli O157:H7. The amount of the released agents from films containing antimicrobial microcapsules was higher than that required for the most resistant microorganism
E. coli O157:H7 at the level of 5% of each agent after two days and at the level of 10% of each agent after 1 day of storage. The fast release of carvacrol from microcapsules was attributed to its liquid form and noncrystallizable property compared to the solid and crystallizable thymol
[41]. Synergistic activity of different compounds present in thyme oil such as thymol and carvacrol (MIC 0.64 mg/mL), compared with pure thymol (MIC 0.73 mg/mL), increased the antimicrobial activity against
E. coli K12
[13]). Based on the MIC values, the encapsulation of pure thymol and thyme oil in β-CD by freeze drying enhanced the antimicrobial activity (0.37 and 0.47 mg/mL, respectively) compared to free thymol and thyme oil. However, the antimicrobial activity of microencapsulated thymol/β-CD and thyme oil/β-CD inclusion complexes prepared by the kneading method were not improved compared to free ones. The differences among MICs of antimicrobial agent/β-CD inclusions against microorganisms were related to the method applied for inclusion complex synthesis, different steric conformation of the guest molecule and the rate of agent release from CD
[13]. The water solubility of carvacrol encapsulated in CD was improved by increasing both the concentration of hydroxyl propyl-β-CD (HPBCD) in water and the temperature that increased contact between carvacrol and
E. coli K12 and
Salmonella Typhimurium LT2 in the medium. Encapsulated carvacrol showed an improved inhibition growth, ranging from 60 to 74% compared to the free one against both pathogens due to improved availability of hydrophobic EOs in the medium through increased water solubility in HPBCD
[12].
Pure coriander EO, as a biologically active agent, is made up of different amounts of various complex components, including oxygenated monoterpenes such as geraniol, linalool, menthol, citronellol; and monoterpene hydrocarbons such as thymol, carvacrol, guaiacol, limonene and sesquiterpenes. High inclusion efficiency of coriander EO (122 mg g
−1) was achieved with the EO/β-CD mixture ratio of 15:85. The encapsulated EO showed high antimicrobial activity against
Aspergillus niger MIUG M5 and
Penicillium glaucum MIUG M9 strains with an inhibition growth zone of 3.1 and 3.0 mm, being lower than the 7.5 and 6.5 mm obtained for antifungal activity of free oil, respectively. Coriander EO/β-CD complexes could retain up to 41–46% of antimicrobial activity of the free EO
[42]. Antimicrobial properties of encapsulated coriander (
Cariandrum sativum) and parsley (
Petroselinum crispum) EOs in β-CD were investigated separately against Gram-positive and Gram-negative bacteria using the colorimetric broth microdilution method. The MIC values of encapsulated EOs against
Listeria innocua,
Achromobacter denitrificans,
Shewanella putrefaciens,
Enterobacter amnigenus and
Pseudomonas fragi were close to each other and ranged between 10 and 20 mg/mL. The higher resistance of all tested bacteria to encapsulated EOs in β-CD compared to free nisin (MIC: 0.625–2.5 mg/mL) could be attributed to enhanced microbial growth supplied by the carbon source of β-CD as a starch-derived polymer
[43]. However, it has been reported that encapsulated coriander oil in chitosan does not have antibacterial activity. Chitosan capsules containing coriander oil showed lower antimicrobial activity than pure chitosan capsules. According to the authors, covering the surface of capsules with EOs with no activity reduced the antimicrobial activity of chitosan
[38].
Encapsulated EG in β-CD exhibited significant antibacterial activity after application of a thermal process at a temperature of 80 °C for 2 h. EG/β-CD complexes had lower MIC values against
E. coli and
Staphylococcus aureus than pure EG molecules due to enhanced EG solubility in an aqueous medium relating to the hydrophilic properties of cyclodextrins. Moreover, the antibacterial activity of microcapsules containing a high amount of EG (17.08 mmol/L) was higher than other microcapsules with lower agent concentrations (9.68 and 10.90 mmol/L) and pure EG molecules
[11]. The antibacterial activity of solid-state EG/β-CD inclusion complexes has been evaluated through an agar cup–plate method with three different concentrations, including 10.0, 5.0, and 2.5 mg/mL (saturated solution of EG/β-CD powder at room temperature), against
E. coli,
Salmonella paratyphi B and
S. aureus. EG/β-CD complexes showed a clear inhibitory effect only against
E. coli with the best result being obtained for the 10 mg/mL solution, indicating the selective antimicrobial activity of prepared complexes against bacterial strains. Free EG did not have any inhibitory effect against studied microorganisms due to two reasons: (i) the slow diffusion of free EG through agar due to the very low solubility level in water and (ii) the high volatility. Cinnamon bark oleoresin (CO) is used in cakes and confectionary as a flavoring agent from bark essential oil. Oleoresin applications in food products is, however, limited due to diverse drawbacks, including being more concentrated than essential oils and degradation by light, heat and oxygen exposure, which can be solved by the encapsulation process
[40][44]. Different ratios of palm hard fat (PH): palm oil (PO) (100:0, 80:20, 60:40) were tested as carriers for the encapsulation of CO (1–2%) by a spray-chilling method. The antimicrobial activity of all the formulations and both free CO and active solid liquid microparticles against
Candida pseudointermedia and
Penicillium paneum increased over 28 days of storage at 25 and 45 °C. Inhibition zones obtained for free CO against both strains were higher than for encapsulated CO, whereas microparticles formed from saturated and unsaturated lipid mixtures displayed good antimicrobial efficiency
[40]. Increased antimicrobial activity of free and encapsulated CO over storage time could be attributed to an increase in both coumarin and O-methoxy cinnamaldehyde concentrations after a possible degradation of the cinnamic acid. Encapsulation of EOs such as EG and cinnamon bark in β-CD increased their antimicrobial activity at lower concentrations as a result of the enhanced water solubility of these substances, which improves their accessibility to the antimicrobial primary sites located at the membrane and inside the cytoplasm of bacteria
[45]. The antimicrobial properties of free and encapsulated EG, clove bud extract, cinnamon bark extract and a 2:1 trans-cinnamaldehyde:EG within β-CD prepared through freeze drying against
Salmonella enterica serovar
Typhimurium LT2 and
L. innocua have been evaluated. The microencapsulation of cinnamon bark extract in β-CD showed the highest inhibitory effect (59%) on the growth of both studied pathogens (MIC: 166 µg/mL) compared to free cinnamon bark extract (MIC: 400 µg/mL for
S. typhimurium and 500 µg/mL for
L. innocua)
[45].
Encapsulation of various bioactive agents via liposomes is considered a promising method for enhancing the shelf-life of food products, mainly due to the significantly increased antimicrobial activity. Thymol and carvacrol, as monoterpenes, have a stabilization effect on the PC/Chol (cholesterol) liposome membranes. Physicochemical properties of liposomes, such as charge, size and composition, along with compounds present in bacterial cells, have important effects on the antimicrobial activity of monoterpenes. Additionally, cellular transport can be promoted by using liposomal formulation, which can lead to the release of active substances inside the cell. The antimicrobial activity of free and encapsulated EOs derived from
Origanum dictamnus (wild and organically cultivated specimen) pure thymol and carvacrol and carvacrol mixtures with thymol (6/1) and γ-terpinene (3/1) in PC/Chol liposomes (PC/C: 10/2 and 5/1) has been assessed against four Gram-positive bacteria, four Gram-negative bacteria and three human pathogenic fungi
[46]. The diffusion technique revealed two important facts, including the higher effectiveness of pure compounds (thymol and carvacrol) compared to oils and increasing antimicrobial activity upon encapsulation. Despite its encapsulation in a small amount (25.0 × 10
−8 g/mL) in liposomes, the antimicrobial activity of carvacrol was equal or increased compared with pure natural compounds (6.0 × 10
−3 g/mL)
[46].
3.2. Propolis
Propolis, or bee glue, is an adhesive and resinous material collected by honey bees (
Apis mellifera L.) from various parts of plants and buds and leaves of trees. The main property of propolis is its inhibitory activity against microorganisms in addition to many other biological activities, such as antioxidant, anti-inflammatory, anesthetic, anticarcinogenic and anticariogenic properties
[47][48][49]. Owing to its great properties, propolis, as a natural additive, can be safely used in food and pharmaceutical industries despite of some disadvantages, such as its unpleasant taste, off-odor and alcohol solubility
[50], which could be overcome by microencapsulation
[23][31][51][52].
The biological activities of propolis are attributed to the presence of several bioactive compounds, such as phenolic compounds, terpenoids, steroids, amino acids and vitamins
[48]. Propolis extract (PE) displays antimicrobial activity against both Gram-positive and Gram-negative bacteria, indicating its possible use as a natural food preservative
[39][41]. Several research studies have demonstrated that Gram-positive bacteria are more sensitive to PE than Gram-negative ones due to differences in bacterial cell structure
[53][54]. In fact, the high permeability of Gram-positive bacteria cells is related to the presence of 90–95% peptidoglycan in cell walls, thus allowing the penetration of antimicrobial agents into the cell, whereas lipopolysaccharides (LPS) from the outer membrane of Gram-negative bacteria create resistance toward natural antimicrobial active compounds
[39]. Spray-dried microparticles of PE (2.5 and 5.0%
w/v) with pea protein as a carrier (2.0%
w/v) have shown bacteriostatic and even bactericidal effects against the Gram-positive bacteria
Listeria monocytogenes and
S. aureus. Unlike PE, these propolis microparticles did not show any activity against either Gram-negative bacteria
S. Typhimurium or
E. coli [39]. The antimicrobial activity of pure PE was shown to be higher than that of encapsulated PE, which could be due to the lower PE concentration in the microparticles
[23][39][41]. The inhibitory activity of microencapsulated propolis in soy protein isolate–pectin coacervates with encapsulant agents and core concentrations between 2.5 and 5.0 g/100 mL against
S. aureus was about 200–400 µg/mL, and for the free one, this ranged between 50 and 100 µg/mL
[23]. Thus, higher concentrations of propolis should be encapsulated to obtain antibacterial activity comparable to that of the free antimicrobial.
The overall antimicrobial activity of encapsulated propolis is influenced by several factors, such as the type and concentration of the wall material used for encapsulation, the amount of PE substances within the dry particles and the presence of various biological compounds in the pure PE. However, no detectable growth of
S. aureus was found for either free propolis or spray-dried propolis–gelatin microcapsules, confirming that spray drying was effective at preserving the properties of the active agent
[51].
3.3. Antimicrobial Peptides
Generally, antimicrobial peptides (AMP) are composed of 20–50 amino acids possessing a broad spectrum of antimicrobial activity and can be found in a wide variety of life forms, from microorganisms to humans. The net positive charge and the amphipathicity are considered as the main properties of AMPs
[55]. The AMP has the ability to kill microbial pathogens through membrane permeabilization and disruption of biological activity of pivotal cytoplasmic substances including proteins, enzymes and RNA and DNA
[56]. Antimicrobial peptides from bacteria can be produced ribosomally or nonribosomally. Ribosomally biosynthesized peptides are known as bacteriocins, of which only a few have been used in the food industry because of their high salt sensitivity and proteolysis
[57]. Among the studied bacteriocins, such as pediocin, subtilin, lacticin, sakacin, leucocin, enterocin, mutacin and mesenterocin
[58], nisin has been the only bacteriocin recognized as GRAS by the FDA since 1980. Nisin is a 3.4 kDa antimicrobial peptide consisting of 34 amin acids with unsaturated amino acids and lanthionin residues. Nisin A differs from nisin Z by a single amino acid substituting histidine at position 27 in nisin A and asparagine in nisin Z. Additionally, other nisin variants have been identified, such as Q, F and U (Ko et al., 2015). It is produced by certain strains of
Lactococcus lactis sp. lactis and has antimicrobial activity against a wide range of Gram-positive bacteria, including foodborne pathogens such as
L. monocytogenes [59]. This metabolite can also inhibit bacterial growth of heat resistant and/or spore-forming microorganisms, particularly those belonging to the
Listeria, Bacillus and
Clostrodium species found in dairy products and canned foods
[57][60]. However, the antimicrobial activity of free nisin added into food products can be reduced over time as a result of establishing complex interactions with food ingredients such as fats and proteins in addition to the possible proteolytic degradation
[61]. Additionally, the emergence of bacteriocin-resistant bacteria might occur through direct incorporation of nisin in food systems
[62]. Microencapsulation is an appropriate alternative able to overcome limitations of direct bacteriocin application in foods and achieve a sustained release of antimicrobial peptides in delivery systems
[35][63].
The slow release of antimicrobial agents such as beacteriocins from microcapsules can improve their antimicrobial activity by reducing the contact time of agents with some food components, resulting in partial inactivation. The antimicrobial activity of alginate microcapsules containing nisin formed by vibrating technology was evaluated against
Brochothrix thermosphacta 7R1. The residual activity of encapsulated nisin in active microcapsule units per mL (AMU/mL) was measured under various conditions (4 and 20 °C, pH 2.5, 4.5 and 6.0) over 168 h. The best result was achieved for the active microcapsules under storage conditions of 4 °C and pH 6.0. The efflux of nisin from microcapsules at higher temperatures and higher pH can lead to the reduction in AMU/mL. The microencapsulation of nisin had a significant effect on total inactivation of 10
4 and 10
6 CFU/mL resting cells of
Brochothrix thermosphacta 7R1
[35]. The antimicrobial activity of nisin encapsulated in liposomes was assessed against
L. monocytogenes and
E. coli O157:H7. The inhibitory effect of PC and PC/PG 6/4 (mol%) liposomes containing 5.0 or 10.0 µg/mL nisin (with or without EDTA) were almost equal to free nisin plus EDTA against
L. monocytogenes. The highest growth inhibition against
E. coli was obtained for liposome-coencapsulated nisin and EDTA. A consistent release of nisin and EDTA within 48 h was obtained for liposomes with PC/PG 6/4 formulation and other liposomes with a low amount of PC or PG released active agents more slowly
[63]. Structural changes in nisin through β-turns’ formation occurring as a result of interactions between nisin and phospholipid membranes can contribute to increasing the stability and antimicrobial activity against bacteria. Additionally, cholesterol can have a stabilizing effect on liposome composition by reducing membrane permeability and improving cohesive interactions. Addition of cholesterol to liposome composition can contribute to its orientation within the fatty acyl chains of phospholipid molecules, while its hydroxyl group faces toward water interface, thus promoting in vitro and in vivo stability of liposomes
[64].
4. Food Applications of Encapsulated Antimicrobials
The microencapsulation of antimicrobial agents is used to prevent the growth of foodborne pathogens present in food systems without losing food quality and nutritional value. Microencapsulated antimicrobials alone or together with other processes were applied to improve the quality of various food products such as meats, dairy products and vegetables (
Table 2). Moreover, it is possible to increase the food’s shelf-life through applying active antimicrobial packaging based on microencapsulated agents such as EOs
[65].
Table 2. Applications of encapsulated antimicrobials for food preservation.
|
Antimicrobial Agents
|
Wall Material
|
Encapsulation Method
|
Food Products
|
Target Microorganism
|
Main Results
|
References
|
|
Nisin
|
Gum arabic
|
Spray drying
|
Milk
|
L. monocytogenes, B. cereus
|
Spray-dried commercial nisin had an antimicrobial effect under refrigeration (90 days)
|
[66]
|
|
Clove oil
|
Β-CD–porous structure
|
Spray drying
|
Meat products
|
Mold spores
|
Encapsulated clove oil had a strong heat resistance and a high antiseptic effect on meat products
|
[67]
|
|
Grape seed extract/carvacrol
|
Chitosan
|
Ionic gelation
|
Salmon
|
Psycrophilic, mesophilic bacteria, Pseudomonas spp.
|
Prepapred microcapsules increased the shelf-life of refrigerated salmon to 4–7 days of storage
|
[68]
|
|
Coconut shell liquid smoke
|
Dextrin
|
Spray-drying
|
Tilapia meat
|
TPC
|
Formulated microcapsules could reduce quality deterioration on fresh fish meat
|
[69]
|
|
Curcumin
|
Gelatin–porous starch
|
Spray-drying
|
Tofu/bread/cooked pork
|
Mold spores
|
Compared with free curcumin, microcapsules reduced mold spores from (34.4 ± 2.5) to (52.3 ± 4.1)%
|
[70]
|
|
Nisin
|
Zein
|
Spray drying
|
milk
|
L. monocytogenes
|
Capsules were more effective than free antimicrobials in inhibiting the growth of L. monocytogenes in 2% reduced fat milk at 25 °C
|
[19]
|
|
Rosemary EO
|
Modified starch–maltodextrin
|
Spray drying
|
Fresh dough
|
Fungi and yeast
|
The encapsulated rosemary essential oil provided long-term antimicrobial activity when applied to fresh dough
|
[71]
|
|
Nisin
|
Alginate–cellulose nanocrystals (CNC) microbeads
|
Alginate microbead
|
RTE meat
|
L. monocytogenes
|
Microencapsulation of nisin (63 μg/mL) increased the lag phase of bacterial growth up to 28 days
|
[28]
|
|
Nisin Z
|
Proliposome H
|
Liposome
|
Cheddar cheese
|
L. innocua
Lactobacillus spp.
L. casei subsp. casei
|
Nisin-containing liposomes could provide a powerful tool to improve nisin stability and availability in the cheese matrix
|
[72]
|
|
Lactoferrin
|
Corn oil–butter fat–polyglycerol polyricinoleate
|
Emulsification
|
Bologna slices
|
Carnobacterium viridans
|
Microencapsulated lactoferrin had greater antimicrobial activity against Carnobacterium viridans than the free one
|
[73]
|