The conversion of solar energy and water to hydrogen via semiconductor photocatalysts is one of the efficient strategies to mitigate the energy and environmental crisis. Conjugated polymeric photocatalysts have advantages over their inorganic counterparts. Their molecular structures, band structures, and electronic properties are easily tunable through molecular engineering to extend their spectral response ranges, improve their quantum efficiencies, and enhance their hydrogen evolution rates. In particular, covalent triazine-based frameworks (CTFs) present a strong potential for solar-driven hydrogen generation due to their large continuous π-conjugated structure, high thermal and chemical stability, and efficient charge transfer and separation capability.
- covalent triazine-based frameworks
- polymeric photocatalyst
- photocatalysis
- hydrogen generation
1. Introduction
2. Optimization of CTFs for Photocatalytic Hydrogen Generation
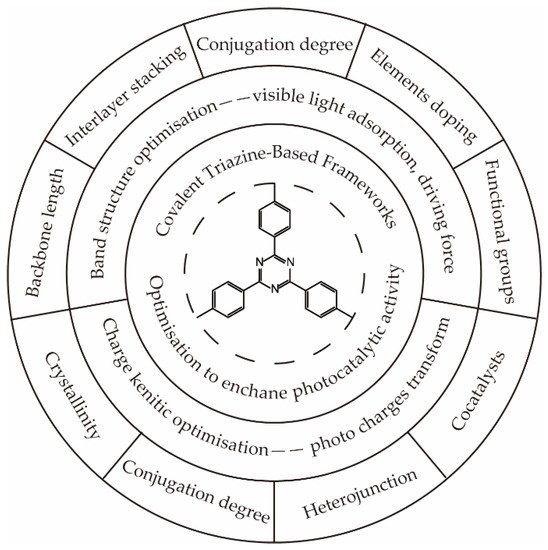
2.1. Optimization of Band Structure to Broaden the Visible Light Absorption and Enhance the Driving Force for Hydrogen Generation
2.1.1. Optimize the Length of the Backbone
2.1.2. Control the Interlayer Stacking
2.1.3. Control the Degree of Polymerization
2.1.4. Element Doping
2.1.5. Functional Group Substitution
2.2. Control of Charge Dynamic to Enhance the Electron Transfer and Separation
2.2.1. Enhance the Degree of Crystallinity
2.2.2. Control the Degree of Conjugation
2.2.3. Heterojunction Construction
2.2.4. Cocatalyst Deposition
This entry is adapted from the peer-reviewed paper 10.3390/polym14071363
