2. Classification of CNS Tumors
There are three main types of glial cells: astrocytes, oligodendrogliocytes, and ependymocytes. According to the type of cells from which a glioma of the brain originates, neurology distinguishes astrocytoma, oligodendroglioma, and ependymoma; there are also mixed gliomas of the brain, such as oligoastrocytomas [
8]. According to the WHO classification, there are four grades (classes) of malignancy of gliomas in the brain [
5]. Grade I gliomas, which are often considered benign, are usually curable by complete surgical resection and rarely, if ever, progress to higher-grade lesions. In contrast, grade II or III gliomas are invasive and progress to higher degrees of lesions. Diffuse grade II and III gliomas are usually less aggressive than higher grade tumors, with a median survival of more than seven years [
8]. There is significant heterogeneity among grade II and III gliomas in terms of pathological features and clinical results. WHO grade IV tumors (glioblastomas), the most invasive form, have the worse prognosis, with a 5-year survival rate of less than 5% after initial diagnosis. After resection of primary glioblastoma, local recurrences may occur in the area of the removed tumor lesion due to the character of tumor growth and formation because of the presence of tumor cells in the adjacent pre-tumor area. Primary glioblastoma develops rapidly without preceding low-grade lesions, while secondary glioblastoma slowly progresses from diffuse or anaplastic astrocytoma (grades II and III according to the WHO classification). Primary and secondary glioblastoma differ genetically rather than histologically [
5,
9].
Different subtypes of gliomas have different aggressiveness spectra and responses to therapeutic treatments. The identification of a particular malignancy into a particular class has long been determined by histological characteristics supported by ancillary tissue-based tests (e.g., immunohistochemical, ultrastructural). However, diagnosis based on histology is subject to much variability between observers. In addition to the diagnostic problems, traditional diagnostic/classification schemes have fallen short of prognostic accuracy, even for patients with the same diagnosis (e.g., grade IV glioblastoma), where survival rates can vary from weeks or months to several years [
9,
10,
11]. In addition, research performed in the last decade has shown that the impact of molecular genetic changes on disease outcome is more significant than some changes in the therapeutic treatment. The development of advanced molecular diagnostic techniques has led to a better understanding of the genomic drivers involved in gliomagenesis and their important prognostic values. The fifth edition of the 2021 WHO Classification of Primary CNS Tumors makes important changes that enhance the role of molecular diagnosis in the classification of CNS tumors [
5,
6]. For each tumor type, different molecular targeted markers are characterized. Taken/evaluated together these molecular markers allow the risk stratification of patients with CNS tumors in terms of prognosis and response to treatment.
3. Telomeres, Telomerase, and the TERT Promoter
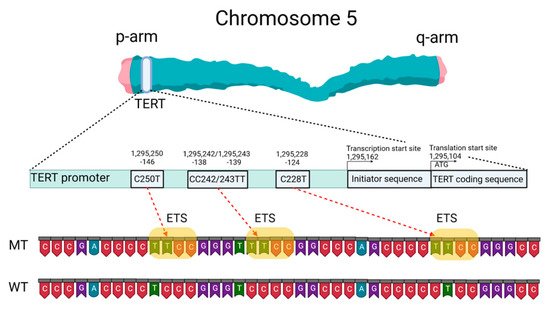
Mutations of the TERTp are known to be absent in normal human cells but are often associated with malignant tumor progression and enhanced proliferation of cells. The two most common mutations in TERTp that are mutually exclusive in CNS tumors are C228T and C250T, located −124 and −146 bp, respectively, prior to the TERT transcription site (chr 51,295,228 C > T and 1,295,250 C > T, respectively, according to GRCh37.p13 genome assembly, 1,295,113 and 1,295,135 according to GRCh38.p13 assembly). The localization of these mutations on the TERTp is shown in Figure 1.
Figure 1. Schematic presentation of TERT gene at chromosome 5p, its promoter structure and two canonical mutations causing gliomagenesis. C > T mutation occurs at one of both positions of the
TERTp (−124 and −146 to ATG for C228T and C250T, respectively) in gliomas, which create de novo ETS binding motifs. CC242/243TT is a rare mutation and has not previously been seen in gliomas; it has been observed in other types of cancer. The figure was created with
BioRender.com (19 March 2021).
These
TERTp mutations create new binding sites for E-26 family transcription factors tryptophan cluster factors class (ETS/TCF) and cause a two- to four-fold increase in transcription of the messenger RNA of the telomerase catalytic subunit [
12]. In addition, among members of this family, mutated
TERTp creates binding sites in CNS tumors for ETS1/2 [
13,
14] and GA-binding proteins (GABP) [
12]. The binding sites for the ETS transcription factor were specified based on the JASPAR CORE database containing a set of profiles derived from published collections of experimentally determined transcription factor binding sites [
15].
The main
TERT promoter does not contain a TATA-box and a CAAT-box, but it includes an array of five GC-boxes surrounded by two E-boxes [
16]. As a result,
TERTp can form a G-quadruplex structure. G-quadruplexes of DNA (G4) are known to be a component of a complex regulatory system in both normal and pathological cells [
17] and can complicate the detection of changes in the primary structure of DNA.
Somatic
TERTp mutations have been observed in various forms of CNS tumors [
18,
19].
TERTp mutations in CNS tumors correlate with the presence of other biomarkers, such as tumor suppressor protein 53 (TP53) gene mutations, isocitrate dehydrogenase 1/2 (IDH1/IDH2) gene mutations, epidermal growth factor receptor (EGFR) changes resulting in overexpression, co-deletion of chromosomal arms 1p and 19q (1p/19q co-deletion), O6-methylguanine-DNA methyltransferase (MGMT) gene promoter methylation status (
MGMTp), and nuclear alpha-thalassemia/mental retardation X-linked syndrome (ATRX) gene mutations [
4]. According to Powter et al., gliomas of low malignancy had the highest rate of co-detection of
TERTp and
IDH1/2 mutations (87.3%), while glioblastomas had a low frequency (joint detection of
TERTp and
IDH1/2 mutations 11.5%). Tumors (
TERTp-mut +
IDH-wt) were significantly associated with
EGFR amplification (44.1%). A total of 54.6% of low-grade gliomas, 71.4% of glioblastomas, and all anaplastic gliomas had
TERTp and phosphatase and tensin homolog (
PTEN) co-mutations.
TERTp-mut and
MGMTp hypermethylation accounted for 51% in low-grade gliomas and 43.6% in glioblastomas.
TERTp-mut is identified in 87.9% of gliomas with a 1p/19q co-deletion.
TERTp-mut is associated with the suppressor/enhancer of Lin-12-like (SEL1L) overexpression. All parameters correlate with overall survival (OS) prognosis [
4].
Mutations in
TERTp causing an increase in telomerase activity and telomere elongation are observed in both the most aggressive form of diffuse glioma, astrocytoma, and the least aggressive form, oligodendroglioma. Hence, telomere maintenance may be a necessary precondition for the formation of CNS tumors [
20]. Considering the use of
TERTp mutations as a diagnostic or prognostic marker of CNS tumors is therefore highly relevant.
4. Telomere Length as a Prognostic Factor for Patients with CNS Tumors
Indirectly, mutations in the TERT gene promoter lead to telomere elongation. Gao and colleagues [
21] measured the relative telomere length of 23 grade I gliomas, which are considered “borderline tumors” (most scientists believe it can be cured after surgery) and showed that telomere length had a significant impact on the survival prognosis for patients. None of the eight patients with short telomere tumor cells died, versus 6 of 15 (40%) patients with long telomeres (died). These results confirm that telomere length may be an important predictor of clinical results in low-grade gliomas of malignancy patients. According to these results, longer telomeres are more typical for gliomas than for meningiomas.
TERTp mutations and longer telomeres were predictors of worse survival for glioma patients regardless of gender, age, severity,
IDH1 and
MGMTp status, radiation therapy, and chemotherapy. Co-detected
TERTp mutations (
TERTp-mut) and telomere elongation are associated with a worse prognosis in patients more frequently than those detected individually. Notably, patients with
TERTp-mut, especially those with C228T, or patients with elongated telomeres, were resistant to radiotherapy. Gao and colleagues revealed that telomere length was significantly shorter in
TERTp-mut cases than in cases with an unchanged promoter sequence (
TERTp-wt) [
21]. Heidenreich and colleagues also showed that telomere length is shorter in gliomas with
TERTp-mut compared to gliomas without
TERTp-mut [
22].
Whether
TERTp mutations are an early or late event in the genesis of CNS tumors has not yet been fully clarified and requires additional investigations. A recent analysis of patients with bladder cancer showed that mutations in
TERTp could be detected in urine samples ten years before the initial clinical diagnosis of bladder cancer. These mutations were absent among comparable control groups that did not develop cancer for 10 years after sampling [
23]. Wang and colleagues detected the mutation in both benign follicular thyroid adenoma and precancerous lesions or follicular tumors with atypical thyroid adenoma or uncertain malignancy potential [
24,
25]. The frequency of
TERTp mutations in the above precancerous lesions was 17%, the same as that of its fully transformed analog of follicular thyroid carcinoma. Importantly, all mutation-carrying malignancies discussed express TERT mRNA and exhibit telomerase activity, which proves that telomerase reactivation occurs within early oncogenic thyroid lesions. Similarly,
TERTp mutation has been identified as an early event in transforming precancerous hepatocellular carcinoma nodules in liver cirrhosis, melanoma, and urothelial papilloma [
26,
27,
28]. At present, it is unclear exactly how
TERTp mutations occur in early oncogenic lesions and completely transformed cells. Although the TERT overexpression level is typically observed in tumors, those with
TERTp-mut have shorter telomeres than non-cancerous tissues. This fact indicates that
TERTp mutations can represent a later event in oncogenesis (second phase) when telomere length has already been depleted [
17,
18]. Another hypothesis suggests that somatic mutations in cells are accumulating at a constant rate throughout life [
29]. Whole genome sequencing data analysis of the Cancer Genome Atlas data of adult diffuse glioma did not show that
TERTp mutations are associated with increased telomere length in grade II–III–IV diffuse gliomas [
30] which argues for an early oncogenic step in this lesion. Abou and colleagues suggested that glioblastoma develops early from a common precursor with loss of at least one copy of the PTEN gene (heterozygous deletion) and a
TERTp mutation: this assumption is based on the high frequency of their co-detection in gliomas [
31].
5. Mutation Status of the TERT Promoter as a Prognostic Marker
The latest edition of the WHO Classification of Primary CNS Tumors in 2021 defined changes in the promoter region of the
TERT gene as one of the key molecular diagnostic markers in the classification of CNS tumors for their treatment [
5,
6,
7]. In three types of primary tumors (oligodendroglioma, glioblastoma, and meningioma),
TERTp-mut is one of the diagnostic parameters. Furthermore, the combined detection of
TERTp-mut and
IDH1/2-mut is considered an alternative feature of oligodendroglioma.
TERTp mutations are the most frequent genomic changes in CNS tumors. Arita and colleagues investigated the presence of mutations in
TERTp in a series of 546 gliomas [
19]. They found a high frequency of mutually exclusive mutations located at common sites, C228T and C250T in all subtypes of the analyzed CNS tumors, of different classes in an average of 55% of all cases. The frequency of mutations was particularly high among primary glioblastomas (70%) and oligodendrogliomas (74%) but relatively low among diffuse astrocytomas (19%) and anaplastic astrocytomas (25%). A similar percentage distribution was shown by a meta-analytic approach (bibliography search) carried out in 2016:
TERTp-mut was frequently found in glioblastoma (69%) and oligodendroglioma (72%), but less frequently in astrocytomas (24%) and oligoastrocytomas (38%) [
32]. Other research has also evaluated the incidence of
TERT mutations in different types of gliomas. Based on these data,
TERT mutations are the most frequently found in glioblastoma (WHO grade IV), oligodendroglioma (WHO grade II), and oligoastrocytoma (WHO grade II), and are also frequently found in diffuse astrocytoma (WHO grade II), anaplastic astrocytoma (WHO grade III), anaplastic oligoastrocytoma (WHO grade III), and anaplastic oligodendroglioma (WHO grade III) [
19,
22,
32,
33,
34,
35,
36,
37,
38]. The data are summarized in
Table 1. In comparison to CNS tumors in adults,
TERTp mutations were exceedingly rare in tumors typically encountered in pediatric patients [
39].
Table 1. Frequency of TERT mutations in different types of gliomas (type of mutation TERTp: C228T and C250T, respectively).
| Authors: |
Arita et al. [19] |
Heidenreich et al. [22] |
Yuan et al. [32] |
Arita et al. [33] |
Pekmezci et al. [34] |
Yang et al. [35] |
Kim et al. [36] |
You et al. [37] |
Huang et al. [38] |
| Diagnosis: |
|
| Diffuse astrocytoma |
19% |
|
|
29% |
|
|
33% |
|
20% |
| Anaplastic astrocytoma |
25% |
|
|
33% |
|
33% |
30% |
32% |
33% |
| Astrocytoma |
|
39% |
24% |
|
22% |
7% |
|
11% |
|
| Glioblastoma |
70% |
80% |
69% |
58% |
66% |
|
64% |
42% |
84% |
| Oligoastrocytoma |
36% |
|
38% |
49% |
|
54% |
|
54% |
|
| Anaplastic oligoastrocytoma |
40% |
|
|
44% |
|
42% |
|
41% |
|
| Oligodendroglioma |
74% |
70% |
72% |
83% |
96% |
74% |
|
76% |
70% |
Anaplastic
oligodendroglioma |
74% |
|
|
74% |
|
67% |
100% |
53% |
|
| Number of patients in the research |
546 |
303 |
3477 |
758 |
1208 |
377 |
67 |
684 |
204 |
According to the data presented in
Table 1, the molecular profiles of low- and high-grade gliomas have different frequencies of
TERTp mutations regardless of tumor class. For example, the highest frequency of
TERTp mutations was found in glioblastomas (WHO grade IV) with an average frequency of 70%, oligodendrogliomas (WHO grade II) with an average frequency of 77%, and anaplastic oligodendrogliomas (WHO grade III) with an average frequency of 74%. At the same time, the lowest frequency of
TERTp mutations was found in diffuse astrocytoma (WHO grade II) with an average frequency of 25%, anaplastic astrocytoma (WHO grade III) with an average frequency of 31%, oligoastrocytoma (WHO grade II) with an average frequency of 46%, and anaplastic oligoastrocytoma (WHO grade III) with an average frequency of 42% [
19,
22,
32,
33,
34,
35,
36,
37,
38].
The most frequent point mutation among gliomas with
TERTp-mut was C228T, in ¾ of the cases. Although C228T and C250T mutations have been reported to be mutually exclusive in CNS tumors, Nonoguchi and colleagues found C228T and C250T co-mutations in only 1 case among 322
IDH-wt glioblastomas in which mutations in both sites were found, with a frequency of 0.31% (1/322). In this study, authors examined
TERTp-mut in C228T and C250T using a cohort of 358 glioblastoma cases in a population-based study that included 36
IDH-mut glioblastoma cases [
40]. The fact that C228T and C250T mutations are mutually exclusive in gliomas suggests that they are each individually sufficient to play a significant oncogenic role in the pathogenesis of gliomas.
Both C228T and C250T mutations generate identical sequences, provide ETS family transcription factor binding, and are equally effective in enhancing TERT transcription. In vivo, the −124 C > T mutation was associated with higher TERT expression in glioblastoma [
12]. This may indicate that the ETS/TCF binding site at the −124 position provides a more favorable/available access point for the transcriptional machinery [
12]. Thus, despite similar far-reaching effects, the two canonical
TERTp mutations can distinctly alter the biology of TERT expression. The mechanism(s) mediating the induction of TERT transcription in cells carrying these mutations remains poorly understood. Perhaps the two
TERTp mutations generating the same ETS binding site are functionally different in the sense that C250T, unlike C228T, is similarly controlled by noncanonical NF-κB signal transduction [
13].
It is also known that these mutations are absent in benign tumors and in tissues of healthy individuals [
2]. Akyerli et al. identified several clinical correlations of
TERTp-mut in patients with gliomas [
41]. Mutations were present in more than half (52.7%) of patients, and
TERTp-mut C228T patients showed lower OS compared to
TERTp-mut C250T patients.
TERTp-muts were found to be homogeneously present in the tumors but not in the surrounding brain parenchyma.
TERTp-mut tumor status did not change over time despite adjuvant therapy or recurrence. The above allows considering
TERTp mutation status as a reliable diagnostic and prognostic factor for CNS tumors.
Hewer and colleagues proposed a technique combining
IDH1/IDH2 and
TERTp (C228T, C250T) mutations assays to distinguish diffuse gliomas from reactive gliosis. The
TERTp mutation assay was successfully applied to distinguish gliomas from gliosis for older adults.
TERTp mutations were not detected in any of the 58 (0%) reactive gliosis samples and in 91 of 117 (78%)
IDH wild-type gliomas. Furthermore, based on a series of 200 consecutive diffuse gliomas, they found that the
IDH mutation assay only had a sensitivity of 28% to detect gliomas, whereas the combined assay yielded a sensitivity of 85% [
42].
A correlation between the occurrence of
TERTp mutation and OS in patients with glioma was reported [
34,
41]. Generally, for patients with high malignancy glioma, the group with
TERTp-mut has a significantly worse OS compared to the
TERTp-wt group. However, gliomas harboring
TERTp mutations are often classified as grade IV gliomas because they initially have a worse predicted OS: only 39 tumors out of 406 (9.6%) in the Eckel-Passow and colleagues research were grade II or III [
20]. When the cohort was considered only for glioblastoma (the most aggressive form of glioma), the following was observed: patients with
TERTp-mut had a shorter OS (11 months) compared to patients with
TERTp-wt (20 months) [
43]. Nonoguchi and colleagues showed that
TERTp-mut status had no effect on OS in glioblastomas when adjusted for other genetic changes and that the prognostic value of
TERTp mutations was largely due to their inverse correlation with
IDH1 mutations [
40]. In low-grade gliomas, the prognostic value of the
TERTp mutation clearly depends on the mutational status of
IDH1/2. Yang and colleagues reported that the
TERTp mutation is a prognostic factor for good OS in grade II/III gliomas, 70–90% of which harbor
IDH mutations [
35]. Some inconsistency in the assessment of the prognostic value of
TERTp mutations may be due to insufficient cohort size or different treatment procedures in the evaluated cohorts. For example, the presence of
TERTp-mut is strongly associated with diagnosis at an older age, which in itself is a well-known prognostic factor and influences treatment decisions.
Summarizing this section, can
TERTp mutation status be considered an independent biomarker of primary glioma? Currently, this question cannot be answered definitively. A potential negative independent prognostic impact of
TERTp mutations was identified; the deleterious effect of
TERTp-mut is correlated with the presence of associated molecular and clinical factors, such as older age,
IDH-wt status, and
MGMTp hypermethylation [
44]. On the other hand, it is noteworthy that
TERTp mutations are a significant prognostic marker in other cancers (e.g., melanoma, thyroid cancer, urothelial carcinoma) and are independent of other mutations. The currently known data show that the prognostic impact of the presence of
TERTp-mut in CNS tumors depends largely on the context of the histological and genomic background of the tumor, primarily the
IDH status [
45], and the methodology for determining mutations in the promoter region of the
TERT gene.