Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Plant Sciences
The UDP-glycosyltransferase 72 family (UGT72) of plants has been shown to glycosylate mainly two classes of phenylpropanoids, (i) the monolignols that are the building blocks of lignin, the second most abundant polymer after cellulose, and (ii) the flavonoids, which play determinant roles in plant interactions with other organisms and in response to stress.
- UGT72 family
- glycosylation
- flavonoid
- monolignol
- coumarins
1. Introduction
In order to adapt to terrestrial environments and biotic interactions, plants have developed the capacity to produce a wide range of specialized compounds. These compounds can undergo various chemical modifications, such as hydroxylation, methylation, or glycosylation. This capacity has been allowed by the diversification of enzymes able to add new functional groups to molecules. Among these modifications, the glycosylation reaction can occur on –OH, –COOH, –NH2, –SH, and C–C groups in diverse molecules such as proteins, carbohydrates, primary and specialized metabolites, as well as xenobiotics [1]. Glycosylation reaction can be carried out with one or several sugars (oligosaccharides) moieties from activated donors, which generate a great diversity of glycosylated compounds. This conjugation reduces the toxicity of the substrate and increases its stability and its solubility owing to the high polarity of the sugar moiety [1]. Glycosylated compounds are stored mainly in the vacuole and their activity may be restored by a single deglycosylation step when required [2,3]. In addition, glycosylation of molecules can modify their interaction with signal receptors, transport proteins, or degradation systems [4,5].
The ubiquitous glycosyltransferases (GTs) are classified in 114 superfamilies on the basis of the similarity of their amino acid sequences [6,7]. The proportion of GT genes in each GT superfamily differs among organisms in function of the peculiarities of their metabolism [8]. GT1, comprising UGTs, is the largest GT superfamily in plants and clusters enzymes glycosylating low molecular weight molecules and making a β-bond using UDP-sugar as sugar donor. UGTs represent about 25% and 35% of GT genes in Arabidopsis thaliana and in Oryza sativa, respectively [8]. The diversity of UGTs allows plants to produce and regulate a myriad of specialized metabolites [9,10].
Plant UGTs can glycosylate phytohormones such as abscisic acid, auxins, brassinosteroids, and salicylic acid, as well as terpenoids. However, most of their substrates derive from the phenylpropanoid pathway whose precursors are the aromatic amino acids phenylalanine and tyrosine (Figure 1) [11]. Besides being building blocks for proteins, these two amino acids are also precursors of a large number of plant products with specific functions in plant growth, development, and stress response [12,13]. Phenylalanine and tyrosine are generated by the shikimate pathway in bacteria, fungi, and plants [12,14]. In plants, the shikimate pathway takes place mainly in the chloroplastic compartment and the produced amino acids are exported to the cytosol [12,13]. The first step of the phenylpropanoid pathway is the deamination of phenylalanine by the phenylalanine ammonia-lyase (PAL) producing cinnamic acid. The second step is the hydroxylation of cinnamic acid at the C4 position (aromatic nucleus) by cinnamate 4-hydroxylase (C4H) leading to p-coumaric acid [15]. Alternatively, tyrosine can be deaminated by bifunctional phenylalanine/tyrosine ammonia-lyase (PTAL) in some plant species, which produces both cinnamic acid from phenylalanine and p-coumaric acid from tyrosine [16]. During the third step, a coenzyme A (CoA) is added to p-coumaric acid by a 4-coumarate: CoA ligase (4CL) to form p-coumaroyl-CoA [15,17,18], which is the last common precursor of monolignols, stilbenes, coumarins, and flavonoids (Figure 1) [19]. In addition, phenylpropenes are synthetized by acetylation and subsequent reduction of monolignols [20]. All these compounds may be glycosylated by UGTs [11]. Monolignol biosynthesis and polymerization have been extensively studied because they are the main components of lignin [2]. In that context, monolignol glycosylation by UGTs may regulate their flux to the cell wall and therefore lignification.
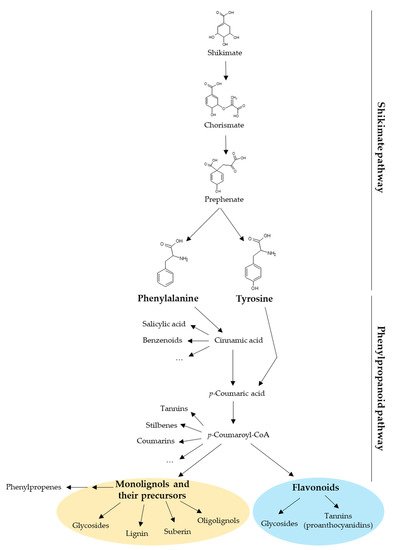
Figure 1. Diversity of compounds produced by the shikimate and the phenylpropanoid pathways in plants. The shikimate pathway yields phenylalanine and tyrosine, which are the precursors of phenylpropanoids. This review focuses on two phenylpropanoid families: the monolignols (yellow circle) and the flavonoids (blue circle). CoA, coenzyme A.
UGTs are classified into families in which members share more than 45% of amino acid sequence identity [21]. However, the prediction of substrate specificity based on primary sequence is complex as divergent families can recognize common substrates and closely related UGTs can have different substrate affinities [5,22]. X-ray crystal three-dimensional (3D) structures of a number of plant UGTs have been reported [23,24] and show that their secondary and tertiary structures are conserved [5,25,26].
2. Functional Characteristics of the UGT72 Family
2.1. Substrate Identification of UGT72s
UGT72 members recognize flavonoids, monolignols, and their precursors/derivatives as substrates. In addition, several of them O/N-glycosylate xenobiotics such as chlorophenols and chloroanilines. UGT phylogeny, based on sequence identities and substrate specificities of the enzymes, is not correlated as diverging UGTs can recognize identical substrates. However, the analysis of the 3D structure of the enzyme may predict the substrate specificity [5].
Concerning monolignols and/or their precursors or derivatives, the A. thaliana UGT72E1 and UGT72E2 use coniferaldehyde and sinapaldehyde, and UGT72E2 and UGT72E3 glycosylate ferulic acid, sinapic acid, caffeic acid, coniferyl alcohol, and sinapyl alcohol [58]. In addition, UGT72E2 can also glycosylate p-, m-, and o-coumaric acid, as well as vanillin [58,59].
Concerning flavonoids, the most frequently reported in vitro substrates for UGT72 members are the flavonols quercetin, kaempferol, and myricetin. However, the glycosylation pattern can be different among UGT72s, as LjUGT72AH1, LjUGT72Z2, LjUGT72V3, GmUGT72X4, and GmUGT72Z3 form 3-O-glycosides [65,66], while MtUGT72L1 forms 3′-O-glycoside [67]. In addition, LjUGT72AD1 forms both 3-O-glycosides and 7-O-glycosides, while CsUGT72AM1 and HpUGT72B11 perform multi-site glycosylation on 3, 7, and 4′ position [60,66,68].
2.2. Possible Roles of UGT72s in Monolignol Homeostasis and in the Regulation of Lignification
2.2.1. Monolignol Homeostasis
The role of UGT72s in monolignol homeostasis has been investigated in A. thaliana and in poplar. In leaves of A. thaliana, the overexpression of UGT72E2 and UGT72E3 induces the accumulation of both coniferin and syringin as compared to the wild type where they are not or poorly detected, while the overexpression of UGT72E1 induces a small accumulation of coniferin. Similarly, in light-grown roots, the overexpression of UGT72E2 and UGT72E3 induces an increase in both coniferin and syringin content as compared to the wild type, and the overexpression of UGT72E1 doubles the amount of coniferin [36,37]. The downregulation of UGT72E2 induces a decrease in monolignol glucosides content in the light-grown roots as compared to the wild type[36]. However, the simultaneous downregulation of the three Arabidopsis UGT72E results in a more pronounced reduction of both coniferin and syringin content than the single UGT72E2 downregulation, suggesting possible redundancy of their function [36]. Furthermore, the overexpression of UGT72E2 and UGT72E3 in Arabidopsis induces a decrease in the amount of sinapoyl malate, as well as the accumulation of ferulic acid glucoside in leaves. In addition, the overexpression of UGT72E3 induces an accumulation of sinapic acid glucoside.
The overexpression of UGT72B1 induces an increase in coniferin content. However, the knock-out mutant of UGT72B1 also accumulates more coniferin than the wild type [39]. This unexpected result was linked to an increase in the expression of genes of the phenylpropanoid pathway in this mutant. Moreover, UGT72B3 and UGT72E2 are also upregulated in the ugt72b1 mutant and could compensate for the UGT72B1 defection [39]. In that case, UGT72B1 may partake in the gene expression regulation of the phenylpropanoid pathway. In addition, the mutant exhibits an accumulation of anthocyanins in the shoot tips which is explained by the upregulation of two important genes of the flavonoid biosynthesis, CHS and DFR [39]. Hence, UGT72B1 appears to have a role in both monolignol and flavonoid homeostasis.
In poplar (P. tremula × P. alba), the overexpression of UGT72AZ1 and UGT72AZ2 triggers the accumulation of coniferin, and the overexpression of UGT72AZ1 causes also the accumulation of syringin. However, the corresponding recombinant proteins do not use monolignols. In contrast, UGT72B37 and UGT72B39 glycosylate monolignols in vitro, but the overexpression of the corresponding genes in poplar does not result in a higher accumulation of monolignol glucosides [61]. These differences in substrate specificity in vitro and in vivo may be due to the substrate availability in planta.
2.2.2. Regulation of Lignification
The involvement of monolignol glucosylation in lignification has been frequently discussed [80,81,82,83]. On the one hand, this process may have a role in monolignol transport in the cell. The mechanisms of subcellular monolignol transport are still under debate, and indirect experiments have evidenced both passive and ATP-dependent transport [80,81,84,85]. Molecular dynamic simulations support that most of the phenylpropanoids involved in lignification can passively cross the membranes, but not their glycosylated derivatives [81]. An ATP-dependent transport of both monolignols and monolignol glucosides has also been evidenced in isolated membrane vesicles from Arabidopsis [80]. As shown by these authors, only aglycon monolignols can cross the plasma membrane, while only glycosylated monolignols can cross the tonoplast, suggesting that monolignol glucosylation may determine the allocation of monolignols in cells [80]. On the other hand, monolignol glycosides may be directly incorporated into lignin as shown by biomimetic in vitro assays (dehydrogenative polymerization catalyzed by horseradish peroxidase in the presence of coniferin and syringin with or without commercial almond β-glucosidase) and nuclear magnetic resonance (NMR) spectroscopy analysis of cell wall and lignin fractions of wood from gymnosperms and angiosperms [83]. This incorporation could possibly intervene into the lignin–carbohydrate complex (linkages between lignin and hemicelluloses/cellulose), which adds complexity for the understanding of the role of glycosylation in the lignification process [83].
Candidates for lignin polymerization regulation have been searched among Arabidopsis and poplar UGT72s that glycosylate monolignols and/or their precursors. In Arabidopsis, GUS assays showed that pUGT72E2- and pUGT72E3-driven expressions are associated with vascular tissues in seedlings (roots, cotyledons, and apical meristem) and in flowers. In addition, pUGT72E2-driven expression is associated with vascular tissues in leaves [37]. pUGT72B1-driven expression was mainly found in the developing xylem, the pith, and the cortex of young floral stems while it is only expressed in the xylem of old floral stems[39]. These results suggest that UGT72E2, UGT72E3, and UGT72B1 may be associated with vascular tissue development, and possibly with lignification. In addition, the transcriptomic analysis of the triple lac4 lac11 lac17 Arabidopsis mutant characterized by a highly disrupted lignin deposition revealed a relation between the expression of UGT72Es and that of laccases (LAC) and peroxidases (PRX) associated with lignin polymerization. In the leaves of this mutant, coniferin and syringin contents are 4-fold higher than the wild type, and the expression of both UGT72E2 and UGT72E3 is 5.2-fold and 2-fold increased, respectively [86]. Another transcriptomic study showed that Arabidopsis transgenic lines overexpressing MYB58 or MYB63 upregulate monolignol biosynthesis genes as well as UGT72E2. These transgenic lines accumulate monolignol glucosides and display ectopic lignification in the epidermis, cortex, and pith [87]. Finally, PRX49 and PRX72, two genes encoding peroxidases likely involved in lignification [88,89], are co-expressed with UGT72E1/UGT72E2 and with the three UGT72Es, respectively [90].
Functional characterization of mutants has evidenced phenotypes to clarify this question. Although single ugt72e1, ugt72e2 or ugt72e3 Arabidopsis mutants do not show any difference in lignin quantity in the floral stem when using the acetyl bromide method, a higher proportion of lignin in the xylem and interfascicular fiber cell walls of the ugt72e3 mutant was evidenced by Raman microspectroscopy and safranin O ratiometric imaging technique, as compared to the wild type [40]. This difference was only observed in the young part and not in the old part of the floral stem and indicates a role of UGT72E3 in cell wall lignification during vascular cells development. Moreover, a higher capacity of incorporation of the three fluorescently labeled monolignols into lignin was found in the ugt72e3 young stem as compared to the wild type. This phenotype was related to an increased expression of lignin-specific PRX71 and LAC17 [40]. The ugt72b1 mutant exhibits a higher lignin content in the whole floral stem, as well as an ectopic lignification in the interfascicular fibers and the pith as compared to the wild type and the rescued line (insertion of the UGT72B1 cDNA under the control of the native gene promoter) [39]. In addition, the mutation leads to a 4-fold increase in the thickness of the pith cell wall. Like monolignol biosynthesis genes, genes potentially involved in monolignol transport across the plasma membrane and in lignin polymerization were upregulated [39]. The authors suggest that the UGT72B1 mutation, which depletes the content of monolignol glucosides, may trigger a signal upregulating the genes involved in monolignol biosynthesis, transport, and polymerization, generating an overproduction of monolignols, hence an increased and ectopic lignification. Consequently, the increase in coniferin in the mutant may be a secondary effect of this monolignol overproduction [39]. The alteration of the monolignol biosynthesis and of lignification may interfere with other developmental processes, explaining the repression of the shoot growth of the ugt72b1 mutant [39].
2.3. UGT72s Involved in Flavonoid Homeostasis
Flavonoids have many roles in plants, especially in stress responses and development [91]. As several UGT72s glycosylate flavonoids, they can be critical in the regulation of these processes. Indeed, glycosylation may modify the activity, transport, accumulation, and biosynthesis of flavonoids [92]. For example, Pang et al. (2008) have demonstrated in vitro that the M. truncatula UGT72L1 can 3′-O-glycosylate epicatechin and epigallocatechin [67]. Epicatechin and epigallocatechin (as well as catechin) are the main components of proanthocyanidins, or condensed tannins which are found in fruits, flowers, bark, and seeds of many plants and whose astringency is known to protect plants against pathogens and herbivores [93]. Moreover, they provide UV protection and antioxidant activity to the plant and are involved in defense against biotic and abiotic stresses [94]. In the ugt72l1 mutant, the reduction of both epicatechin and epicatechin 3′-O-glucoside content triggers a decrease in extractable proanthocyanidins in seeds. UGT72L1 could regulate proanthocyanidin biosynthesis by directing the flux of epicatechin into the vacuole [95]. Indeed, the MATE1 transporter, which is involved in the epicatechin transport across the tonoplast like its Arabidopsis homologous TT12, has been demonstrated to be specific to epicatechin 3′-O-glucoside [96]. Moreover, as suggested by Pang and colleagues [67], this glycosylation may protect the plant against free epicatechins and help to direct monomers polymerization into the accurate 4–8 linkage.
The majority of UGT72s glycosylating flavonoids in vitro have not been studied for their role in stress tolerance. However, a transcriptomic analysis reveals, for instance, that UGT72B3 is upregulated in Arabidopsis aerial parts when temperature decreases from 20 °C to 4 °C, while UGT72E1 is downregulated in the same conditions. UGT72E1 and UGT72D1 are upregulated in roots after infection by Plasmodiophora brassicae. UGT72D1 is downregulated during drought [97].
Flavonoids are also involved in developmental processes, especially by interacting with hormone signalings [91,99] and UGT72s may also be involved in those processes. In L. japonicus, UGT72AD1 and UGT72Z2 glycosylate in vitro kaempferol, quercetin, and myricetin, both forming 7-O-glycosides, while 3-O-glycosides were detected for UGT72AD1 only [66]. They are mainly expressed during the later stages of seed development in a similar manner to FLS, MYB11, and MYB14, which are involved in flavonoid biosynthesis [66]. The overexpression of UGT72AD1 and UGT72Z2 in L. japonicus hairy roots does not significantly modify the total flavonoid content. However, the flavonol content is increased in all of the transgenic lines compared to the wild type. Especially, kaempferol 3-O-rhamnoside-7-O-rhamnoside, kaempferol 3-O-glucoside-7-O-rhamnoside, and two additional flavonol hexosides are more accumulated.
2.4. The Subcellular Localization of UGT72s Provides Information on Their Functions
UGT72s have been localized in different subcellular compartments. For instance, UGT72L1 fused to the green fluorescent protein (GFP) was detected in the cytosol in M. truncatula [95]. The first plant UGT localized into the nucleus was UGT72E1-GFP which interacts with the Arabidopsis MAP kinase kinase kinase SIS8, involved in sugar signaling [100]. In poplar, five UGT72s fused to GFP (UGT72AZ1, UGT72AZ2, UGT72B36, UGT72B37, and UGT72B39) are localized both in the nucleus and associated with the endoplasmic reticulum (ER). The localization of UGT72s in the nucleus may reveal a specialization of these UGTs in the phenylpropanoid homeostasis in this specific compartment. There is no report of monolignol glycosides in the nucleus and their potential function. However, some flavonoids are localized in the nucleus in several plant species, suggesting that this localization is also possible for monolignols [101,102,103]. It has been proposed that flavonoids may interfere with some nuclear proteins involved in DNA organization, signaling pathway, and gene expression [103,104,105], and these processes may be regulated by UGT72s.
The Polygonum tinctorium indoxyl-β-D-glucoside synthase (IGS), a member of the UGT72B sub-family, was localized in both cytosolic and microsomal fractions, suggesting a reversible binding of the protein to the membranes. The ER localization was confirmed using ultracentrifugation with a sucrose density gradient [106]. The Pyrus bretschneideri PbUGT72AJ2 fused to GFP was also located mainly in the cytosol and cytomembrane compartment [107]. The enzymatic steps of the phenylpropanoid pathway are known to occur in the cytosol and several enzymes of the monolignol biosynthesis pathway, such as HCT and the P450 proteins C4H, C3′H, and F5H are, at least partly, associated with the ER [108,109]. Assembly and disassembly of a given metabolon, for instance, a UGT coupled to a P450 protein would provide additional flexibility in specialized metabolite biosynthesis. Finally, UGT72A2-GFP is localized in the chloroplast [61]. The chloroplast localization of UGT72A2 fits with its function in flavonoid and ROS homeostasis within the chloroplast. Chloroplasts are indeed storage and even biosynthesis organelles for some phenylpropanoids such as kaempferol, quercetin, and catechin in different plant species [110,111,112]. These compounds may be involved in ROS scavenging in this compartment [113]. The subcellular localization of the majority of UGT72s has not been investigated yet, which could improve our knowledge of the biological functions in this family.
This entry is adapted from the peer-reviewed paper 10.3390/biology11030441
This entry is offline, you can click here to edit this entry!
