Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Developmental Biology
Adventitious root (AR) formation is required for the vegetative propagation of economically important horticultural crops, such as apples. Asexual propagation is commonly utilized for breeding programs because of its short life cycle, true-to-typeness, and high efficiency. The lack of AR formation from stem segments is a barrier to segment survival.
- apples
- adventitious root (AR)
- formation
- asexual reproduction
- sugars
- polyamines
1. Role of Phenolic Compound in the Regulation of ARs
The involvement of phenolic compounds in AR formation has been well established for a long time [21,22,35]. These were shown to either synergize or inhibit the activity of auxin [95]. Phenolic compounds keep plants from oxidative stress [96]. Besides shielding auxins from oxidation, phenolic compounds have been associated with them in various ways. Flavonoids may also limit auxin transport [97]. Flavonoids either interact with PIN2 or have an impact on the extent of PIN proteins [98]. The role of phenolic compounds was tested on Jork 9 stem slices during AR formation. The results suggested that all orthodiphenols, paradiphenols, and triphenols investigated with IAA enhanced adventitious rooting from stem slices. The most effective treatment was ferulic acid (FA) (a methylated orthodiphenol), which raised the number of ARs from 0.9 to 5.8. There was little or no improvement with NAA following the inclusion of phenolics. FA and phloroglucinol (a triphenol) were studied in-depth. Based on their effects on the IAA dose–response curve and the duration of their activity, both acted as antioxidants, preventing IAA decarboxylation and oxidative stress in the tissue [21]. Auxin degradation includes oxidative decarboxylation via peroxidases, but since phenolics influence peroxidase activity, auxin catabolism at the cuttings’ base may be prevented [21]. After wounding, there is an elevation in JA, auxin, and phenolic compounds at the cutting base during the AR induction stage, with a decrease in peroxidase activity. However, peroxidase activity increases to a peak during the initiation stage, and the auxin concentration decreases [35,84].
2. Role of Sugars in the Regulation of ARs
Sugars are both energy sources and signaling molecules that control plant growth. The published articles related to sugar and apple ARs are gathered in Table 1. The detailed studies of sugar metabolism focusing on the anatomical changes in apple rootstock Jork 9 were conducted by Jasik and De Klerk [23]. As highlighted in the initial developmental stage, a large number of starch grains were found in the cells that started AR primordium formation at the stem base, and thus the percentage of plastids grew dramatically, with the starch grains occupying a major share of all apparent plastids at the same stage. The utilization of sugars produced by the hydrolysis of all of these starch grains provides energy for adventitious rooting, which is associated with an increase in the number of cambial mitochondria, dictyosomes, and nuclei to the visible detriment of vacuolar expansion. Moreover, Pawlicki and colleagues [24] used different combinations and concentrations of carbohydrates and auxin to investigate AR differentiation. They found that sucrose (29–50 mM) stimulated adventitious rooting but also supported callus formation. In contrast, sorbitol, which is required in large quantities for optimal adventitious rooting, was not highly supportive of callus formation. Furthermore, the combinations of sucrose and mannitol (59/29 mM) or glucose and sorbitol (117/59 mM) ensued in 100% AR formation, with more than 6 ARs per disc. Then, in the presence of the sucrose-mannitol combination, the discs were treated with 49.2 µM of IBA for 540 min, obtaining an adventitious rooting of 100%.
The number of ARs was affected by sucrose concentration, although the effect was minor across a wide range of sucrose concentrations (1–9%). There was also a synergy between sucrose and auxin, which facilitated adventitious rooting. When slices were cultivated in a sucrose-free medium for 0–2 days, root formation was decreased. However, 2 days of cultivation minus sucrose had no impact or even a minor enhancer effect on later days, suggesting that sucrose is needed as a source of energy and a building block during adventitious rooting in apples [25]. The cuttings of M7 apple rootstock were treated with IBA and found to contain glucose, sorbitol, fructose, and inositol. In the initial 10 days of AR formation, the content of soluble saccharides improved significantly, and this was associated with the rapid cell divisions at the stem base in this stage. Specifically, the amount of fructose content in the stem basal part was related to rooting potential. At the AR primordia differentiation and AR emergence stages, the concentration of soluble saccharides in the cuttings had dropped to its initial level, indicating that soluble saccharides are crucial for the initial stages but not essential for later stages of AR formation in apples [26].
A recent study was conducted on T337, where the IBA treatment induces the expression of sucrose synthase4 (SUS4), sucrose phosphate synthase4 (SPS4), and polyol/monosaccharide transporter (PMT5) at the induction stage [7]. The application of KCl to B9 apple rootstock also upregulates the expression of various starch and sucrose metabolism-related genes [32]. These results show that IBA and KCl had some crosstalk with sugars during the AR induction stage, and sugar was transported into the stem basal parts by the activities of several genes, providing adequate energy and signal activity for ARs to begin.
3. Role of Polyamines in the Regulation of ARs
Polyamines (PAs), including putrescine, spermidine, and spermidine, are a class of organic compounds with two or more main amino acid groups found abundantly in plant cells. Additionally, PAs are important in controlling DNA duplication and cell proliferation, senescence, stress responses, and morphogenesis. They also play a key function in forming and developing root architecture [99]. Exogenous application of spermidine on Malus prunifolia var. ringo stem cuttings increased AR formation by interacting with IAA. Spermidine application upregulated the expression levels of spermidine-related genes (SAMDC1, PAO, and SPDS6) and auxin biosynthesis-related genes (IAA7, IAA14, and IAA23) during AR formation. In contrast, WOX11 upregulated the expression levels of LBD16 and LBD29, which prompted the transcripts of genes related to the cell cycle (CYCD1;1 and CYCP4;1) [27]. Nonetheless, they have been shown to be inhibitors in a number of species, including poplar [100] and walnut [101]. PAs, in conjunction with auxin, modulate cell division and root primordia initiation during the induction stage [102]. Nonetheless, the relationship between PAs and auxin for the rooting process is still lacking.
4. Role of Nutrients in the Life of ARs
Mineral nutrients are necessary for plant metabolism. AR formation and nutrients are closely related. The published articles related to nutrients and apple ARs are gathered in Table 1. The details of nutrients are explained below.
4.1. Role of Nitrogen in the Formation of ARs
Nitrogen is regarded as an important macronutrient that is needed for plant development and higher yields [103]. Nitrogen assimilation in soil occurs in two ways, such as organic and inorganic [104]. Nitrate is a vital nitrogen source that also functions as a signaling molecule for controlling flowering time, AR, and LR formation, as well as prompting auxin-related gene expressions [28,105,106]. Nitrate levels in the soil are relatively low due to its high solubility, leaching capabilities, and fast absorption by bacteria and fungus [107]. In higher plants, such as apples, there are two types of nitrate transport mechanisms: low-affinity transport systems (LATS) and high-affinity transport systems (HATS), which are responsible for uptake, distribution, and storage of nitrate [108]. The nitrate supply is immediately and strongly detected by the plant cells. Following this, the nitrate signaling system changes the relative expression levels of several gene sets to control cell and organ metabolism. Furthermore, the availability of nitrate has a significant effect on AR formation. In general, the external concentration of nitrate generated binary effects on the formation of AR depending on their concentrations, with an activated impact at low levels; however, a limiting effect at high levels was seen in Arabidopsis [109]. A similar phenomenon was also seen in a recent study, where different nitrate concentrations (9.4 mM/L, 18.8 mM/L, 28.1 mM/L, 46.9 mM/L, and 84.5 mM/L) were exogenously treated to B9 apple rootstock stem cuttings during adventitious rooting, and 28.1 mM/L was found to be the most favorable nitrate level for adventitious rooting, and 46.9 mM/L and 84.5 mM/L were found to be inhibitors [28]. High nitrate inhibits AR in apples by elevating the endogenous levels of ABA, ZR, JA, BR, and GA3, which may create a hormonal imbalance in the plant. In addition, the high ratios of IAA/ABA and IAA/ZR promote ARs under nitrate treatments. Furthermore, transcriptome analysis showed that hormone signaling pathway-related genes were upregulated, and root development and cell cycle-related pathways were repressed by the application of high nitrate [31]. Moreover, auxin and ABA signaling miRNAs (miR390a, miR160a, miR167, miR169a, and miR394) were activated, and miRNAs related to cell fate transformation, expansion, and enlargement (miR166, miR171, miR319, miR156, and miR396) were repressed by high nitrate [30]. The same cited authors explained the mechanism of a different set of genes how 28.1 mM/L treatment promotes AR formation in B9 compared with 46.9 mM/L (inhibiting treatment). The results showed that treatment with 28.1 mM/L noticeably upregulated the relative expression levels of nitrate related genes (NRT1.1, NRT2.1, NIA1, and ANR1) and auxin biosynthesis (IAA14 and IAA23), which enhances the AR development-related gene expression (WOX11, ARRO1, and SHR) and collectively induces the expression of cell cycle related genes (CYCD1;1, CYCD3;1, and CYCP4;1) in comparison with 46.9 mM/L nitrate treatment [29]. NRT2.1 a high affinity nitrate transporter showed the highest response to nitrate availability, indicating that NRT2.1 may play a key role in forming AR in apples, and the overexpression of MdNRT2.1 gene in tobacco produced superior roots compared to WT plants.
Ammonium, such as nitrate, played an important role in root development in apple and other crops [110,111,112]. The effects of nitrate and ammonium were studied in apples, where significant differences were detected in root morphology within a week of application. The roots were thin and long in response to nitrate treatment, although they were thick and short in response to ammonium application, with prominent enlarged areas behind the tip. Furthermore, nitrate-treated roots were nearly devoid of root hairs, but those treated with ammonium were entirely covered in thick, long root hairs, and the root hair cylinder diameter in the ammonium treated was around three times that of the nitrate treated [111]. Hilo and colleagues [112] found that ammonium-treated cuttings (without nitrate) had a higher total nitrogen content, which was indicated by enhanced glutamine and asparagine levels. These authors point to faster ammonium assimilation in the stem base, which could have resulted in a lower expression level of N-regulated genes such as the ammonium transporter AMT1 [112]. Moreover, the effects of ammonium nitrate (NH4NO3) and potassium nitrate (KNO3) were also studied in three apple scion cultivars [110]. The percentage ARs of Gala and Royal Gala rose dramatically when the level of NH4NO3 in the medium was reduced from full strength to 1/4 strength, but not in Jonagold. A further decrease in NH4NO3 concentration from 1/4 strength to zero considerably decreased the rate of ARs in Gala but not in Royal Gala. However, Jonagold rooted optimally in the absence of NH4NO3. Moreover, without NH4NO3, adventitious rooting for all three cultivars was as high as 100% when KNO3 was given at full strength. These results suggest that the effect of NH4NO3 was cultivar-specific, but KNO3 treatment at full strength promoted ARs in all cultivars.
4.2. Role of Potassium in the Formation of ARs
Potassium (K) is an indispensable macronutrient, and it is the most abundant cation absorbed by higher plants. It comprises more than 10% of the plant’s total dry weight [113]. Its decline to below 10 g/kg of dry weight leads to serious growth issues in various plant species. Indeed, despite not being an important element of any structural and functional molecules, it is involved in various key physiological processes, such as metabolism and plant growth and development [114], flowering [115], improved fruit quality [116], and AR formation in apples and other plants [32,117]. In addition, under K deficiency in soil, plant root growth is weakened. As a result, the yields and outcomes are often limited [118]. The uptake and translocation of K is mediated by several K channels and transporters [113,119].
The underlying physiological and molecular mechanisms regulating AR by KCl application were studied in B9 apple rootstock. KCl-treated cuttings produced a significantly higher number of ARs and increased AR length than control cuttings. At most time points during AR formation, KCl promoted several hormone levels, including IAA, ZR, JA, and GA, and decreased the ratios of ABA/JA, ABA/ZR, and ABA/IAA. Moreover, transcriptome analysis revealed that KCl promoted ARs through the auxin signaling pathway and sugar metabolism and by increasing the genes related to AR development and cell cycle [32]. Z.R. Zhao and colleagues found the promotive effect of K on the formation of ARs in cucumber cotyledons and mung bean hypocotyls, as well as in kidney beans [117]. The results of these studies indicated that the K application promotes adventitious rooting in apples and other crops.
The effect of nitrate and potassium treatments on the various endogenous hormones during adventitious rooting in apples is shown in Figure 2.
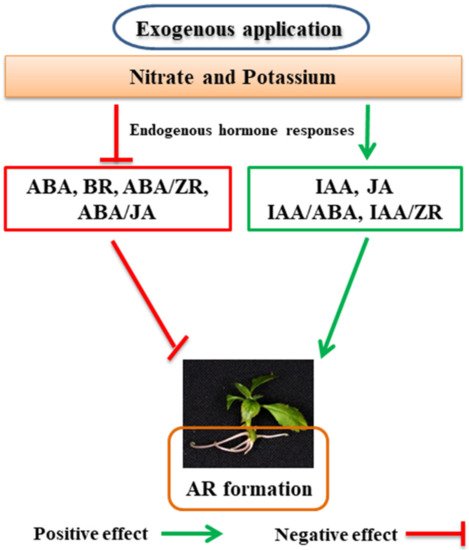
Figure 2. A suggested schematic diagram of how exogenous application of nitrate and potassium regulates different endogenous hormones during the formation of adventitious roots (ARs) in apples. Indole-3-acetic acid (IAA), zeatin ribosome (ZR), jasmonic acid (JA), abscisic acid (ABA), and brassinosteroid (BR). Ratios of hormone content, such as ABA/ZR, ABA/JA, IAA/ZR, and IAA/ABA. The green color shows a positive effect, and the red indicates a negative effect.
This entry is adapted from the peer-reviewed paper 10.3390/horticulturae8040276
This entry is offline, you can click here to edit this entry!
