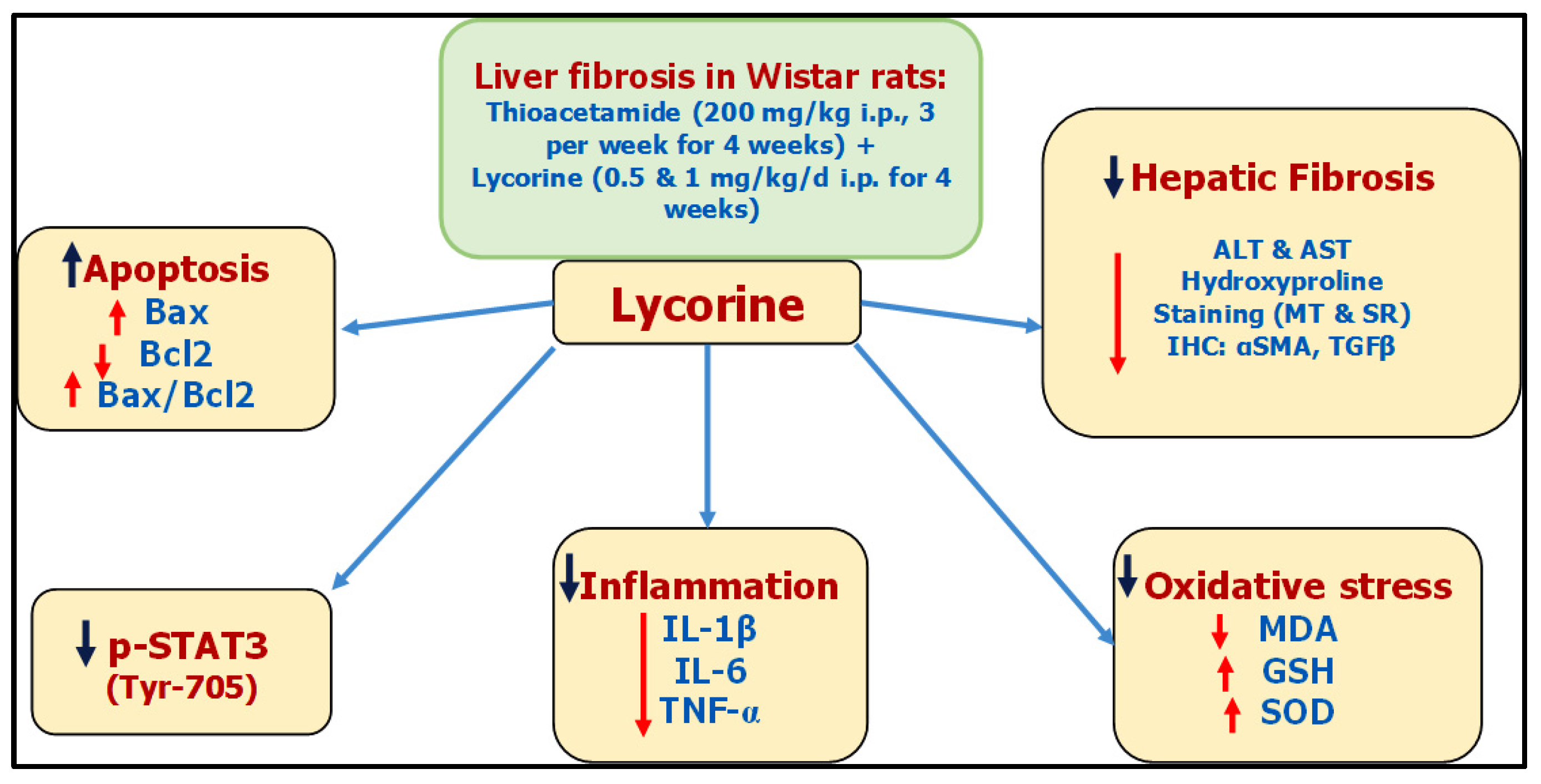
Liver fibrosis is a foremost medical concern worldwide. Lycorine—a natural alkaloid—has antioxidant, anti-inflammatory, and antitumor activates. Lycorine hinders TAA-induced liver fibrosis in rats, due to—at least partly—its antioxidative and anti-inflammatory properties, along with its ability to inhibit Signal Transducer and Activator of Transcription factor (STAT3) signaling.
- lycorine
- thioacetamide
- liver fibrosis
- STAT3
1. Introduction
2. The Potential Antifibrotic Effect of Lycorine against Thioacetamide-Induced Liver Fibrosis in Rats
Presently, there is no reasonable remedy for liver fibrosis [22]. Lycorine is a natural alkaloid that possesses antitumor [13][14], antioxidant [23], and anti-inflammatory activities [24], in addition to being hepatoprotective in CCl4-induced acute hepatotoxicity [16]. Moreover, it has been reported to activate the mitochondrial apoptosis pathway via targeting STAT3 [17].
For decades, TAA has been known for its ability to induce liver fibrosis [25]. Yet, its molecular mechanism is still not fully unstated. Indeed, TAA undergoes bioactivation in the liver via oxidation processes via hepatic CYP2E1 [26][27]. This leads to the formation of reactive metabolites, namely, S-oxide and SS-dioxide, which are apparently responsible for TAA-induced hepatic injury [28]. Basically, liver injury activates hepatic stellate cells (HSCs) to multiply and secrete extracellular matrix (ECM) components like interstitial collagens [29][30]. Moreover, HSCs are converted into myofibroblasts expressing α-SMA, which induces fibrogenesis and tissue stiffness [31]. By mechanisms including autocrine and paracrine pathways, ECM components promote growth factor signaling, principally by TGF-β, which in-turn add to the activation of HSCs, generating a positive feedback circle [32]. It is worth mentioning that the TGF-β family comprises three isoforms TGF-β 1, 2, and 3 with different biological activities [33]. The isoform TGF-β1 gained the most importance due to its pleiotropic nature, extending from tissue fibrosis to preparing the microenvironment for carcinogenesis [34][35]. With regard to hepatic pathogenesis, the assessed TGF-β1 has been reported to have a critical role in hepatic fibrosis through direct activation of HSCs and accumulation of ECM [36]. Lycorine treatment significantly diminished TAA-induced hepatic injury by significantly ameliorating the rise of hepatic transaminases “ALT and AST” and the fibrotic marker “hydroxyproline”. This was confirmed by its ability to improve histopathological-fibrotic changes, as evidenced by H&E, MT, and SR staining, and to guard against the excessive immunohistochemical expression of αSMA and TGFβ1. These findings are in line with recent studies investigating the antifibrotic activity of lycorine against bleomycin-induced pulmonary fibrosis [18], as well as experimentally-induced cardiac dysfunction [19][20][21].
3. The Possible Causal Mechanisms with Respect to the STAT3 Pathway
4. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/ph15030369
References
- Rouiller, C. The Liver: Morphology, Biochemistry, Physiology; Academic Press: Cambridge, MA, USA, 2013.
- Asrani, S.K.; Devarbhavi, H.; Eaton, J.; Kamath, P.S. Burden of liver diseases in the world. J. Hepatol. 2019, 70, 151–171.
- Bataller, R.; Brenner, D.A. Liver fibrosis. J. Clin. Investig. 2005, 115, 209–218.
- Pinzani, M. Pathophysiology of Liver Fibrosis. Dig. Dis. 2015, 33, 492–497.
- Seki, E.; Brenner, D.A. Recent advancement of molecular mechanisms of liver fibrosis. J. Hepato-Biliary-Pancreat. Sci. 2015, 22, 512–518.
- Novo, E.; Parola, M. Redox mechanisms in hepatic chronic wound healing and fibrogenesis. Fibrogenes. Tissue Repair 2008, 1, 5.
- Dauer, D.J.; Ferraro, B.; Song, L.; Yu, B.; Mora, L.; Buettner, R.; Enkemann, S.; Jove, R.; Haura, E.B. Stat3 regulates genes common to both wound healing and cancer. Oncogene 2005, 24, 3397–3408.
- Levy, D.E.; Darnell, J.E. STATs: Transcriptional control and biological impact. Nat. Rev. Mol. Cell Biol. 2002, 3, 651–662.
- Ogata, H.; Chinen, T.; Yoshida, T.; Kinjyo, I.; Takaesu, G.; Shiraishi, H.; Iida, M.; Kobayashi, T.; Yoshimura, A. Loss of SOCS3 in the liver promotes fibrosis by enhancing STAT3-mediated TGF-β1 production. Oncogene 2006, 25, 2520–2530.
- Hirano, T.; Ishihara, K.; Hibi, M. Roles of STAT3 in mediating the cell growth, differentiation and survival signals relayed through the IL-6 family of cytokine receptors. Oncogene 2000, 19, 2548–2556.
- Hiraganahalli, B.D.; Chinampudur, V.C.; Dethe, S.; Mundkinajeddu, D.; Pandre, M.K.; Balachandran, J.; Agarwal, A. Hepatoprotective and antioxidant activity of standardized herbal extracts. Pharmacogn. Mag. 2012, 8, 116–123.
- De Leo, P.; Dalessandro, G.; De Santis, A.; Arrigoni, O. Inhibitory effect of lycorine on cell division and cell elongation. Plant Cell Physiol. 1973, 14, 481–486.
- Liu, X.S.; Jiang, J.; Jiao, X.Y.; Wu, Y.E.; Lin, J.H.; Cai, Y.M. Lycorine induces apoptosis and down-regulation of Mcl-1 in human leukemia cells. Cancer Lett. 2009, 274, 16–24.
- McNulty, J.; Nair, J.J.; Bastida, J.; Pandey, S.; Griffin, C. Structure-activity studies on the lycorine pharmacophore: A potent inducer of apoptosis in human leukemia cells. Phytochemistry 2009, 70, 913–919.
- Oleyede, K.G.; Oke, M.J.; Raji, Y.; Olugbade, T. Antioxidant and Anticonvulsant Alkaloids in Crinum ornatum Bulb Extract. World J. Chem. 2010, 5, 26–31.
- Çitoǧlu, G.S.; Acikara, O.B.; Yilmaz, B.S.; Özbek, H. Evaluation of analgesic, anti-inflammatory and hepatoprotective effects of lycorine from Sternbergia fisheriana (Herbert) Rupr. Fitoterapia 2012, 83, 81–87.
- Wu, S.; Qiu, Y.; Shao, Y.; Yin, S.; Wang, R.; Pang, X.; Ma, J.; Zhang, C.; Wu, B.; Koo, S.; et al. Lycorine Displays Potent Antitumor Efficacy in Colon Carcinoma by Targeting STAT3. Front. Pharmacol. 2018, 9, 881.
- Liang, Q.; Cai, W.; Zhao, Y.; Xu, H.; Tang, H.; Chen, D.; Qian, F.; Sun, L. Lycorine ameliorates bleomycin-induced pulmonary fibrosis via inhibiting NLRP3 inflammasome activation and pyroptosis. Pharmacol. Res. 2020, 158, 104884.
- Schimmel, K.; Jung, M.; Foinquinos, A.; José, G.S.; Beaumont, J.; Bock, K.; Grote-Levi, L.; Xiao, K.; Bär, C.; Pfanne, A.; et al. Natural compound library screening identifies new molecules for the treatment of cardiac fibrosis and diastolic dysfunction. Circulation 2020, 141, 751–767.
- Ni, T.; Huang, X.; Pan, S.; Lu, Z. Dihydrolycorine Attenuates Cardiac Fibrosis and Dysfunction by Downregulating Runx1 following Myocardial Infarction. Oxid. Med. Cell. Longev. 2021, 2021, 8528239.
- Wu, J.; Fu, Y.; Wu, Y.X.; Wu, Z.X.; Wang, Z.H.; Li, P. Lycorine ameliorates isoproterenol-induced cardiac dysfunction mainly via inhibiting inflammation, fibrosis, oxidative stress and apoptosis. Bioengineered 2021, 12, 5583–5594.
- Lam, P.; Cheung, F.; Tan, H.Y.; Wang, N.; Yuen, M.F.; Feng, Y. Hepatoprotective effects of chinese medicinal herbs: A focus on anti-inflammatory and anti-oxidative activities. Int. J. Mol. Sci. 2016, 17, 465.
- Ilavenil, S.; Kaleeswaran, B.; Sumitha, P.; Tamilvendan, D.; Ravikumar, S. Protection of human erythrocyte using Crinum asiaticum extract and lycorine from oxidative damage induced by 2-amidinopropane. Saudi J. Biol. Sci. 2011, 18, 181–187.
- Kang, J.; Zhang, Y.; Cao, X.; Fan, J.; Li, G.; Wang, Q.; Diao, Y.; Zhao, Z.; Luo, L.; Yin, Z. Lycorine inhibits lipopolysaccharide-induced iNOS and COX-2 up-regulation in RAW264.7 cells through suppressing P38 and STATs activation and increases the survival rate of mice after LPS challenge. Int. Immunopharmacol. 2012, 12, 249–256.
- Crespo Yanguas, S.; Cogliati, B.; Willebrords, J.; Maes, M.; Colle, I.; van den Bossche, B.; de Oliveira, C.P.M.S.; Andraus, W.; Alves, V.A.; Leclercq, I.; et al. Experimental models of liver fibrosis. Arch. Toxicol. 2016, 90, 1025–1048.
- Chilakapati, J.; Korrapati, M.C.; Shankar, K.; Hill, R.A.; Warbritton, A.; Latendresse, J.R.; Mehendale, H.M. Role of CYP2E1 and saturation kinetics in the bioactivation of thioacetamide: Effects of diet restriction and phenobarbital. Toxicol. Appl. Pharmacol. 2007, 219, 72–84.
- Kang, J.S.; Wanibuchi, H.; Morimura, K.; Wongpoomchai, R.; Chusiri, Y.; Gonzalez, F.J.; Fukushima, S. Role of CYP2E1 in thioacetamide-induced mouse hepatotoxicity. Toxicol. Appl. Pharmacol. 2008, 228, 295–300.
- Hajovsky, H.; Hu, G.; Koen, Y.; Sarma, D.; Cui, W.; Moore, D.S.; Staudinger, J.L.; Hanzlik, R.P. Metabolism and toxicity of thioacetamide and thioacetamide S-Oxide in rat hepatocytes. Chem. Res. Toxicol. 2012, 25, 1955–1963.
- Mederacke, I.; Hsu, C.C.; Troeger, J.S.; Huebener, P.; Mu, X.; Dapito, D.H.; Pradere, J.P.; Schwabe, R.F. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat. Commun. 2013, 4, 2823.
- Iwaisako, K.; Jiang, C.; Zhang, M.; Cong, M.; Moore-Morris, T.J.; Park, T.J.; Liu, X.; Xu, J.; Wang, P.; Paik, Y.H.; et al. Origin of myofibroblasts in the fibrotic liver in mice. Proc. Natl. Acad. Sci. USA 2014, 111, E3297–E3305.
- Friedman, S.L. Mechanisms of Hepatic Fibrogenesis. Gastroenterology 2008, 134, 1655–1669.
- Ricard-Blum, S.; Baffet, G.; Théret, N. Molecular and tissue alterations of collagens in fibrosis. Matrix Biol. 2018, 68–69, 122–149.
- Voisin, A.; Damon-Soubeyrand, C.; Bravard, S.; Saez, F.; Drevet, J.R.; Guiton, R. Differential expression and localisation of TGF-β isoforms and receptors in the murine epididymis. Sci. Rep. 2020, 10, 995.
- Prud’homme, G.J. Pathobiology of transforming growth factor β in cancer, fibrosis and immunologic disease, and therapeutic considerations. Lab. Investig. 2007, 87, 1077–1091.
- Chung, J.Y.F.; Chan, M.K.K.; Li, J.S.F.; Chan, A.S.W.; Tang, P.C.T.; Leung, K.T.; To, K.F.; Lan, H.Y.; Tang, P.M.K. TGF-β Signaling: From Tissue Fibrosis to Tumor Microenvironment. Int. J. Mol. Sci. 2021, 22, 7575.
- Xu, M.Y.; Hu, J.J.; Shen, J.; Wang, M.L.; Zhang, Q.Q.; Qu, Y.; Lu, L.G. Stat3 signaling activation crosslinking of TGF-β1 in hepatic stellate cell exacerbates liver injury and fibrosis. Biochim. Biophys. Acta-Mol. Basis Dis. 2014, 1842, 2237–2245.
- Ilavenil, S.; Karthik, D.; Arasu, M.V.; Vijayakumar, M.; Srigopalram, S.; Arokiyaraj, S.; Ravikumar, S.; Choi, K.C.; Ravikumar, S. Hepatoprotective mechanism of lycorine against carbon tetrachloride induced toxicity in swiss albino mice—A proteomic approach Keywords: Lycorine CCl 4 Oxidative stress 2D gel MALDI-TOF-MS ATP synthase Regucalcin HSP 60. Asian Pac. J. Reprod. 2015, 4, 123–128.
- Robert, S.; Gicquel, T.; Bodin, A.; Lagente, V.; Boichot, E. Characterization of the MMP/TIMP imbalance and collagen production induced by IL-1 β or TNF-α release from human hepatic stellate cells. PLoS ONE 2016, 11, e0153118.
- Mikami, M.; Kitahara, M.; Kitano, M.; Ariki, Y.; Mimaki, Y.; Sashida, Y.; Yamazaki, M.; Yui, S. Suppressive activity of lycoricidinol (narciclasine) against cytotoxicity of neutrophil-derived calprotectin, and its suppressive effect on rat adjuvant arthritis model. Biol. Pharm. Bull. 1999, 22, 674–678.
- Zhao, J.; Qi, Y.F.; Yu, Y.R. STAT3: A key regulator in liver fibrosis. Ann. Hepatol. 2021, 21, 100224.
- Choi, S.; Jung, H.J.; Kim, M.W.; Kang, J.H.; Shin, D.; Jang, Y.S.; Yoon, Y.S.; Oh, S.H. A novel STAT3 inhibitor, STX-0119, attenuates liver fibrosis by inactivating hepatic stellate cells in mice. Biochem. Biophys. Res. Commun. 2019, 513, 49–55.
- Lopez, O.N.; Bohanon, F.J.; Wang, X.; Ye, N.; Corsello, T.; Rojas-Khalil, Y.; Chen, H.; Chen, H.; Zhou, J.; Radhakrishnan, R.S. STAT3 Inhibition Suppresses Hepatic Stellate Cell Fibrogenesis: HJC0123, a Potential Therapeutic Agent for Liver Fibrosis. RSC Adv. 2016, 6, 100652.
- Sallam, A.M.; Esmat, A.; Abdel-Naim, A.B. Cucurbitacin-B attenuates CCL4-induced hepatic fibrosis in mice through inhibition of STAT-3. Chem. Biol. Drug Des. 2018, 91, 933–941.
- Fan, J.; Chen, Q.; Wei, L.; Zhou, X.; Wang, R.; Zhang, H. Asiatic acid ameliorates CCL4-induced liver fibrosis in rats: Involvement of Nrf2/ARE, NF-κB/iκBα, and JAK1/STAT3 signaling pathways. Drug Des. Dev. Ther. 2018, 12, 3595.
- Gong, Z.; Ye, H.; Huo, Y.; Wang, L.; Huang, Y.; Huang, M.; Yuan, X. S-allyl-cysteine attenuates carbon tetrachloride-induced liver fibrosis in rats by targeting STAT3/SMAD3 pathway. Am. J. Transl. Res. 2018, 10, 1337–1346.
- Lin, I.Y.; Chiou, Y.S.; Wu, L.C.; Tsai, C.Y.; Chen, C.T.; Chuang, W.C.; Lee, M.C.; Lin, C.C.; Lin, T.T.; Chen, S.C.; et al. CCM111 prevents hepatic fibrosis via cooperative inhibition of TGF-β, Wnt and STAT3 signaling pathways. J. Food Drug Anal. 2019, 27, 184–194.
- Liu, J.; Hu, W.X.; He, L.F.; Ye, M.; Li, Y. Effects of lycorine on HL-60 cells via arresting cell cycle and inducing apoptosis. FEBS Lett. 2004, 578, 245–250.
- Li, L.; Dai, H.-J.; Ye, M.; Wang, S.-L.; Xiao, X.-J.; Zheng, J.; Chen, H.-Y.; Luo, Y.-H.; Liu, J. Lycorine induces cell-cycle arrest in the G0/G1 phase in K562 cells via HDAC inhibition. Cancer Cell Int. 2012, 12, 49.
- He, J.; Hong, B.; Bian, M.; Jin, H.; Chen, J.; Shao, J.; Zhang, F.; Zheng, S. Docosahexaenoic acid inhibits hepatic stellate cell activation to attenuate liver fibrosis in a PPARγ-dependent manner. Int. Immunopharmacol. 2019, 75, 105816.
- Koda, Y.; Teratani, T.; Chu, P.-S.; Hagihara, Y.; Mikami, Y.; Harada, Y.; Tsujikawa, H.; Miyamoto, K.; Suzuki, T.; Taniki, N.; et al. CD8+ tissue-resident memory T cells promote liver fibrosis resolution by inducing apoptosis of hepatic stellate cells. Nat. Commun. 2021, 12, 4474.
