Electrospinning is a well-known technique to produce fibers that mimic the three dimensional microstructural arrangements of the extracellular matrix fibers. Natural and synthetic polymers are used in the electrospinning process; moreover, a blend of them provides composite materials that have demonstrated the potential advantage of supporting cell function and adhesion. Recently, the decellularized extracellular matrix (dECM), which is the noncellular component of tissue that retains relevant biological cues for cells, has been evaluated as a starting biomaterial to realize composite electrospun constructs. The properties of the electrospun systems can be further improved with innovative procedures of functionalization with biomolecules. Among the various approaches, great attention is devoted to the “click” concept in constructing a bioactive system, due to the modularity, orthogonality, and simplicity features of the “click” reactions. Here, we provide an overview of current approaches that can be used to obtain biofunctional composite electrospun biomaterials and propose a design of a smart ECM-based electrospun system suitable for skeletal muscle tissue regeneration.
- electrospinning
- decellularized extracellular matrix (dECM)
- click chemistry
- skeletal muscle regeneration
1. Introduction
The development of regenerative biomaterials and the progress in their processing represent key factors to generate smart biomimetic scaffolds resembling the structural organization and activity of native tissue in order to guide tissue regeneration [1,2]. Nowadays, researchers aim to develop tissue-specific scaffolds characterized by desired topographical mechanical and physical features. Among them, the skeletal muscle represents a complex and challenging tissue to be generated in vitro for tissue engineering purposes. Scaffolding strategies aim to create ideal scaffolds with mechanical, chemical, and biological properties that mimic the composition and the structure of extracellular matrix (ECM) of native tissue to encourage cell adhesion and proliferation [7,8]. The ECM represents the major structural component of the human body, and it is composed of a three-dimensional arrangement of natural polymers such as collagen, elastin, and fibrinogen as well as a mixture of macromolecules such as growth factors, peptides, glycoproteins, and proteoglycans. However, the ECM is not only a structural framework for tissues but through interactions with receptors on the surfaces of cells, it plays an important role in both day-to-day cellular activity and in tissue regeneration [9,10]. For these reasons, the engineering of an ECM-mimicking scaffold is extremely challenging. In the native tissues, most ECM components consist of interwoven fibrous structures in the micronanoscale range and thus, the fabrication of scaffolds mimicking ECM structural organization is an active area of research in TE. To date, phase separation, self-assembly, and electrospinning have been used to make scaffolds with a fibrous network [11]. Among these techniques, electrospinning has continued to be the most commonly used. Electrospinning is a powerful and scalable production method [12], which allows the fabrication of micro- or nanofibers possessing large surface areas and high porosity, which are favorable for biomedical applications in terms of cellular interactions [13,14]. In particular, to control the fiber arrangement, topography, morphology, and the overall performance of electrospun polymeric scaffolds for TE applications, different electrospinning processing factors, such as applied voltage, tip of needle-collector distance, solution viscosity, and feed rate need to be optimized [17]. The role of main effects and interactions between process parameters can be effectively captured by regression and design of experiments methods [18,19]. The orientation of fibers is an important feature of an ideal and promising scaffold because this aspect greatly influences cell growth and related functions in cells such as nerve and smooth muscle cells [20]. In particular, the creation of polymeric aligned electrospun biomaterials that mimic skeletal muscle allows the efficient organization of muscle cells to form aligned myotubes during muscle regeneration [3]. Over the past two decades, electrospun scaffolds have been obtained using natural polymers, synthetic polymers, or a combination of those [21,22,23] according to the desired combination of mechanical and chemical properties that ultimately dictate the biological response toward the targeted tissue regeneration application. Recently, the development of the decellularization processes of organs and tissues has made possible the creation of an electrospun scaffold prepared from processing methods of a native extracellular matrix. The decellularized extracellular matrix (dECM) is an organ or a tissue devoid of its cellular content but which preserves a rather large portion of its original composition including its architecture, structural organization, and biochemical cues [24,25]. This dECM material can be used as a scaffold that maintains its original geometry directly in medical interventions or can be repopulated with cells before use. More recently, dECMs have been further processed to generate dECM products that can be used as a starting material for biofabrication techniques such as electrospinning [26]. Indeed, electrospun dECM scaffolds are typically achieved by adding components of dECM after the electrospinning process or by direct electrospinning dECM components [27]. However, during processing to obtain the dECM materials, some key components such as glycosaminoglycans (GAGs) can be lost. Indeed, GAG concentrations are especially important for their interaction with growth factors and chemokines, influencing cell signaling [28]. To overcome these obstacles, some authors are developing ad hoc decellurization protocols for specific tissues and the optimization of subsequent dECM process methods with the aim of avoiding the loss of some important bioactive components of the native ECM [29,30]. Another effective way to ensure the bioactivity of the electrospun scaffolds is the judicious combination of electrospun fibers with appropriate biomolecules, such as small molecules, growth factors, short peptides, or proteins [31], in order to stimulate a specific cell response. In this context, the development of innovative and forefront electrospinning techniques and an accurate choice of polymeric materials and biomolecules enables the loading of bioactive components during the manufacturing process. A local and controlled release of biomolecules enhances the functional properties of scaffold and influence surrounding tissue regeneration. Furthermore, the regeneration process is strongly influenced by the interactions between cells and biomaterial surfaces. For this reason, the postelectrospinning modification of the high surface of fibers with biomolecules represents a suitable method in order to enhance cell adhesion and organization [22,32]. Such postelectrospinning modifications can be achieved by the physical adsorption or chemical bonding of biomolecules to the fibers. In this context, the biofunctionalization of the fiber surface using “click” reactions has drawn significant interest recently, owing to their simple reaction conditions, high reaction rate, and high chemical selectivity [33,34].
In this paper, we discuss current approaches that can be used to obtain functional composite electrospun biomaterials with a focus on the possible strategies to achieve bioactive scaffolds. The main steps discussed concern (i) obtaining composite electrospun biomaterials with particular attention to the use of native ECM in the solution or fusion of electrospinning; (ii) the incorporation of bioactive molecules during electrospinning (bulk functionalization) and the surface modification of electrospun systems through biomolecules; (iii) biofunctionalization postprocessing using “click” reactions, as schematically represented in Figure 1. Finally, we designed a strategy for dECM-based composite bioactive electrospun scaffolds to promote skeletal muscle regeneration.
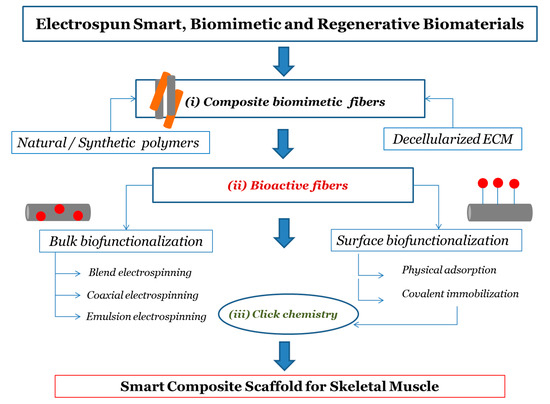
2. Development of composite and bioactive electrospun biomaterials
Natural and synthetic polymers have been widely used to realize scaffolds through Electrospinning technologies. Despite the excellent biodegradability, chemical, and mechanical properties of electrospun nanofibrous scaffolds from synthetic polymers, they often require further modification to their surface and structure to promote their biofunctionality, since synthetic biomaterials lack bioactive functional sites and result in poor biochemical similarities with ECM [37]. Conversely, compared to synthetic polymers, natural polymers offer intrinsic similarity to ECM components and bring with them a biological signature that is advantageous to support cell adhesion and proliferation [8,21,38]. Collagen, gelatin, chitosan, hyaluronic acid, cellulose, and glycans in general have in fact been proposed to produce electrospun fibers in TE because of their biocompatibility and low immunogenicity [21,39]. However, these scaffolds usually lack load-bearing capability due to lower mechanical strength and higher degradation rates than synthetic polymers [21,22]. A blend of polymers can be an attractive option to mix the benefits and overcome the limitations of the individual one-component systems described above. In particular, the combination of natural and synthetic polymers is a means to effectively improve the biocompatibility, mechanical, and structural properties of the scaffold to boost cell adhesion and growth and to promote tissue regeneration [41]. In recent years, the research in scaffold engineering has shifted from using natural polymers to using directly the component of the decellularized extracellular matrix (dECM) to obtain scaffolds mimicking native ECM [53]. In particular, dECM from different tissues of origin may be processed in order to obtain a suitable product that can be directly loaded into an electrospinning polymeric solution to fabricate composite scaffolds that combine the versatility of polymeric materials and the biological complexity of natural ECM. In addition, the properties of the composite electrospun systems can be further enhanced with innovative procedures of functionalization with biomolecules. Bioactive components can be both internalized (encapsulated) into the fibrous scaffold during the electrospinning and attached on the fiber surface in a postprocessing step. Among the various methods, great attention is dedicated to the “click” concept in constructing bioactive scaffolds, due to the modularity, orthogonality, and simplicity features of the “click” reactions.
3. Customized Functionalization by Click Chemistry of Composite dECM-Based Electrospun Scaffold for Skeletal Tissue Regeneration
Skeletal muscle is an important body-composition component in humans and plays a key role in voluntary movement and locomotion [85]. In addition, skeletal muscle is involved in other physiological processes, including thermogenesis, metabolism, and the secretion of numerous peptides for communication with other tissues [85]. For these reasons, the maintenance of skeletal muscle health is of vital importance. Although skeletal muscle is highly regenerative following injury or disease, endogenous self-regeneration is harshly impaired in the conditions of severe volume traumatic muscle loss (VML). Consequently, a growing demand arose in skeletal muscle TE to fully restore the structure and function of lost muscle [86]. The structure of muscle tissue is composed of oriented muscle fibers (myofibers), which are embedded into an extracellular matrix (ECM) consisting of many components such as collagen, glycoproteins, proteoglycans, and elastin [87]. In addition, ECM, through its components such as GAGs, bind and store various growth factors which influence cell behavior and regulate cell proliferation, migration, and differentiation [87]. The skeletal muscle engineering approaches focus on the design and development of a fibrous scaffold with appropriate mechanical, morphological, and biofunctional properties to facilitate muscle growth and regeneration [87]. Among the most important properties that biomaterials should possess for successful skeletal muscle TE include: porosity, aligned architecture, and bioavailability of bioactive molecules to promote the cell activity [3]. To this end, electrospinning is a forefront method to produce a fibrous scaffold that could mimic the structure and high anisotropic organization of native muscle tissue [88]. In particular, the composite electrospun scaffold could assure biocompatibility, biodegradability, controlled mechanical properties, and high porosity due to the synergistic effects of the different components. Furthermore, advances in decellularization protocols of muscle tissue allow the preservation of the important components of ECM and the realization of potential dECM-based scaffolds that could provide the correct support for myofiber development and the appropriate architecture to form muscle regeneration. In order to optimize the biological properties of the composite electrospun scaffolds, biomolecules can be opportunely selected to stimulate skeletal muscle regeneration. In this context, coupling electrospinning and click reactions appears to be a valid method to design biofunctionalized fibrous scaffolds promising for skeletal muscle repair.
4. Conclusions
In recent years, the decellularized extracellular matrix (dECM) has been demonstrated to be a suitable and promising starting material to fabricate composite electrospun scaffolds. In addition, the biofunctionalization of the electrospun fibers represents an interesting approach for the further exploration of the potential of electrospinning. In particular, the bioactivity of electrospun scaffolds could be assured by the covalent immobilization of biomolecules via click reaction in order to promote cell adhesion and proliferation. The combination of electrospinning and click reactions represents a promising strategy to design and develop leading and smart biomaterials systems for tissue engineering applications. In perspective, this strategy could be a valid starting point for obtaining customizable and intelligent biomaterial systems that are able to successfully guide the regeneration process of skeletal muscle.
This entry is adapted from the peer-reviewed paper 10.3390/nano10091781
