The thermochemical water-splitting method is a promising technology for efficiently converting renewable thermal energy sources into green hydrogen. Thermochemical water splitting uses a high-temperature source, e.g., nuclear energy, waste heat, or concentrated solar systems, to convert water into hydrogen and oxygen through cyclic chemical reactions. Several promising routes have been proposed for hydrogen production through green technologies such as biological processes (such as CO gas-fermentation, dark fermentation, etc.), electrical (such as alkaline electrolysis cell, anion exchange membrane electrolysis cell, proton exchange membrane electrolysis cell, solid oxide electrolysis cell, etc.), photonic (bio-photolysis, photofermentation, etc.), thermochemical, etc.
- thermochemical water splitting
- green hydrogen production
- redox loop
1. Introduction
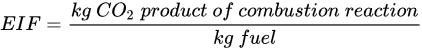 (1)
(1)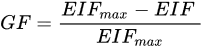 (2)
(2)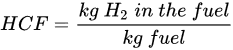 (3)
(3)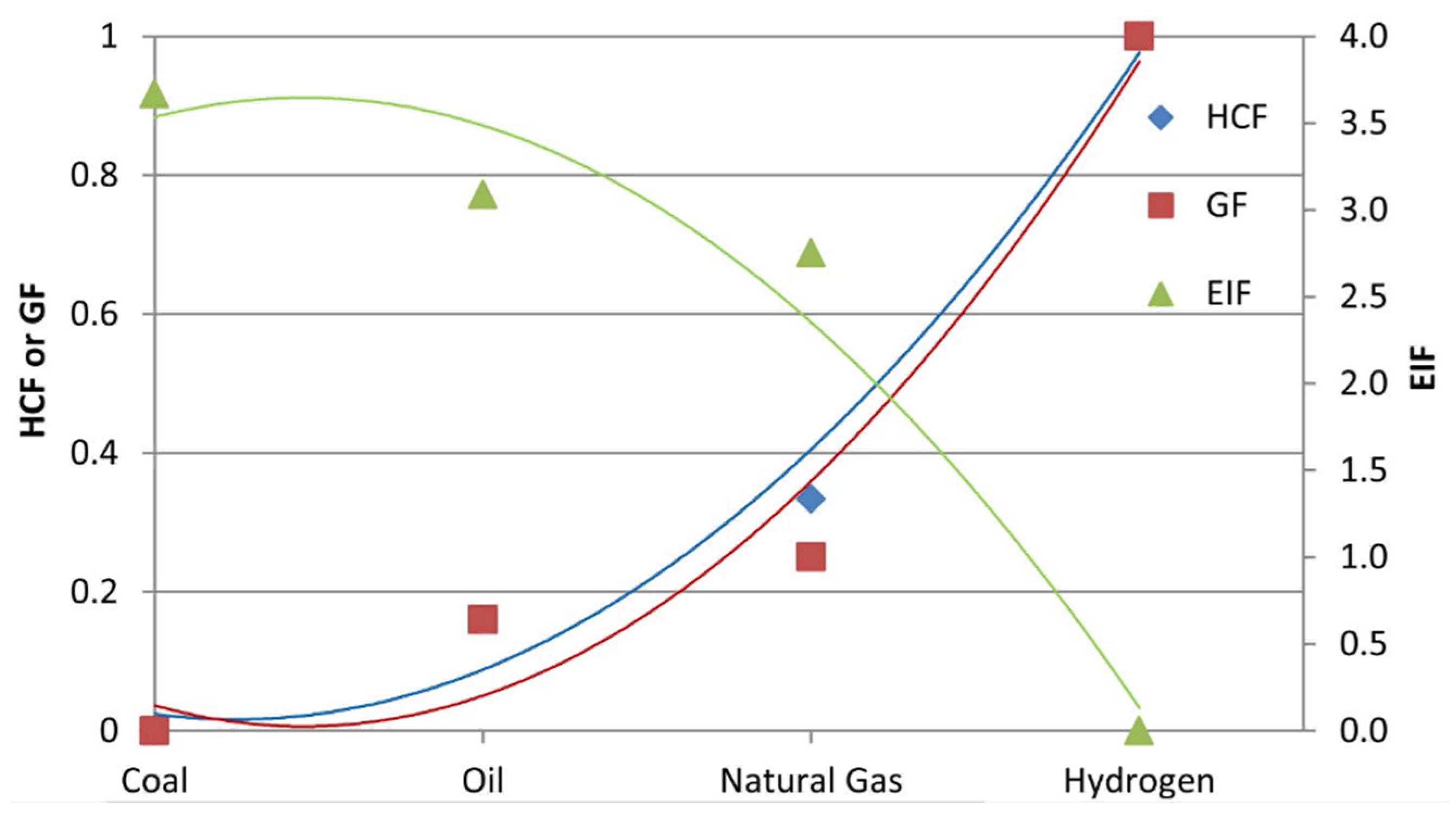 Figure 1. HCF, GF, and EIF of coal, oil, natural gas, and hydrogen (Reproduced with permission from [17], Elsevier: 2015).
Figure 1. HCF, GF, and EIF of coal, oil, natural gas, and hydrogen (Reproduced with permission from [17], Elsevier: 2015).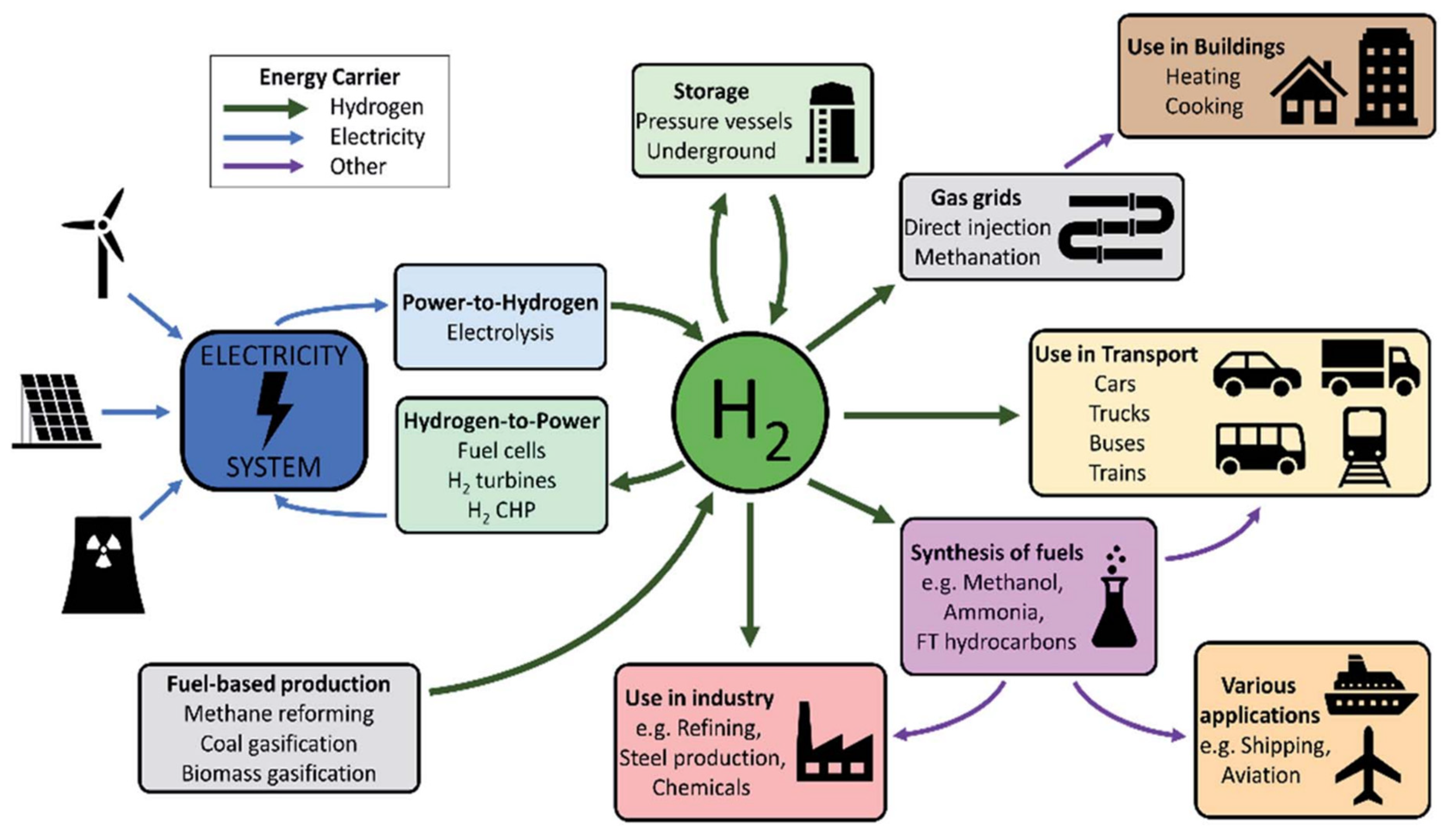 Figure 2. A summary of hydrogen applications [30].
Figure 2. A summary of hydrogen applications [30].2. Green Hydrogen Technologies
- -Low-temperature cycles with an operating temperature below 1100 °C, such as sulfur-iodine, hybrid sulfur, hybrid copper chloride, etc.
- -High-temperature cycles with an operating temperature above 1100 °C, such as Zn/ZnO, FeO/Fe3O4, manganese oxide-based, ferrite cycles, etc. [98]
-
3. Summary
Optimal energy strategy plays an essential role in improving process optimization to increase lifetime and cost-effectiveness. Energy and exergy analysis of an S-I cycle coupled with HI-I2-H2O electrolysis has been conducted by Ying et al. [110]. The whole cycle was divided into three sections, i.e., Bunsen reaction, sulfuric acid decomposition, and HI-I2-H2O electrolysis. According to the results, the energy and exergy efficiencies of the system were about 15.3–31.0% and 32.8%, respectively. Due to the transformation of electricity to exergy in an electrolytic cell, sulfuric acid decomposer and condenser and Bunsen reaction sections accounted for about 93.0% and 63.4% of the exergy destruction, respectively. The highest exergy efficiency of the proposed S-I cycle was about 32.8% which was lower than the four-step Cu-Cl cycle (75.7% [246]) and Zn/ZnO cycle (~40%) [247]. The highest energy efficiency was also about 31.0%, which was lower than the Cu-Cl (41.9% [246]) and Zn/ZnO cycles (~40% [192]), implying that more studies should be conducted to improve the overall energy and exergy efficiencies of the proposed cycle such as appropriate waste heat recovery and internal heat exchange was recommended by the authors.Juárez-Martínez et al. [91] optimized the energy efficiency of an S-I cycle using a new network consisting of four heat exchangers and a heuristic-ruled method. The effect of the new network of heat exchangers on the system’s efficiency was analyzed, and it was found that the energy needed for cooling decreased. Thus, the overall thermal energy efficiency improved by about 10%.Wang et al. [248] analyzed the energy efficiency of direct solar hydrogen using low-cost materials. As the current hydrogen production systems suffer from either low efficiencies or expensive materials, they claimed that their proposed hydrogen generation system comprised of catalysts and low-cost light-absorbers could reach 20% efficiency of solar-to-hydrogen (STH) conversion by coupling high-performance perovskite-Si tandem cells with earth-abundant catalysts. The electrodes were made of high-density NiMo with flower-stem morphology with minimal reaction overpotential (~6 mV at 10 mA·cm−2) and increased reaction sites. Enhancing the performance of perovskite cells with an open-circuit voltage of 1.271 V can improve surface passivation and, consequently, surge the overall efficiency from 20% to 25%. In another attempt, Jia et al. [249] reported a higher hydrogen efficiency of over 30% using photovoltaic-electrolysis technology for water splitting. The system includes an InGaP/GaAs/GaInNAsSb triple-junction solar cell in series with two polymeric electrolyte membrane electrolyzers. Since the mentioned assembly produced a high voltage to drive the electrolyzers, no extra input power was used in the system. The operating capacity of the electrolyzers was well matched to the maximum output power of the photovoltaic cell by adjusting the solar concentration. A schematic of the PV-electrolysis is shown in Figure 3. High STH efficiency of about 30% was reported for the PV-electrolysis system. It is interesting to note that using two electrolyzers in series with a high-efficiency solar cell minimized the required additional voltage, thus improving the water splitting efficiency.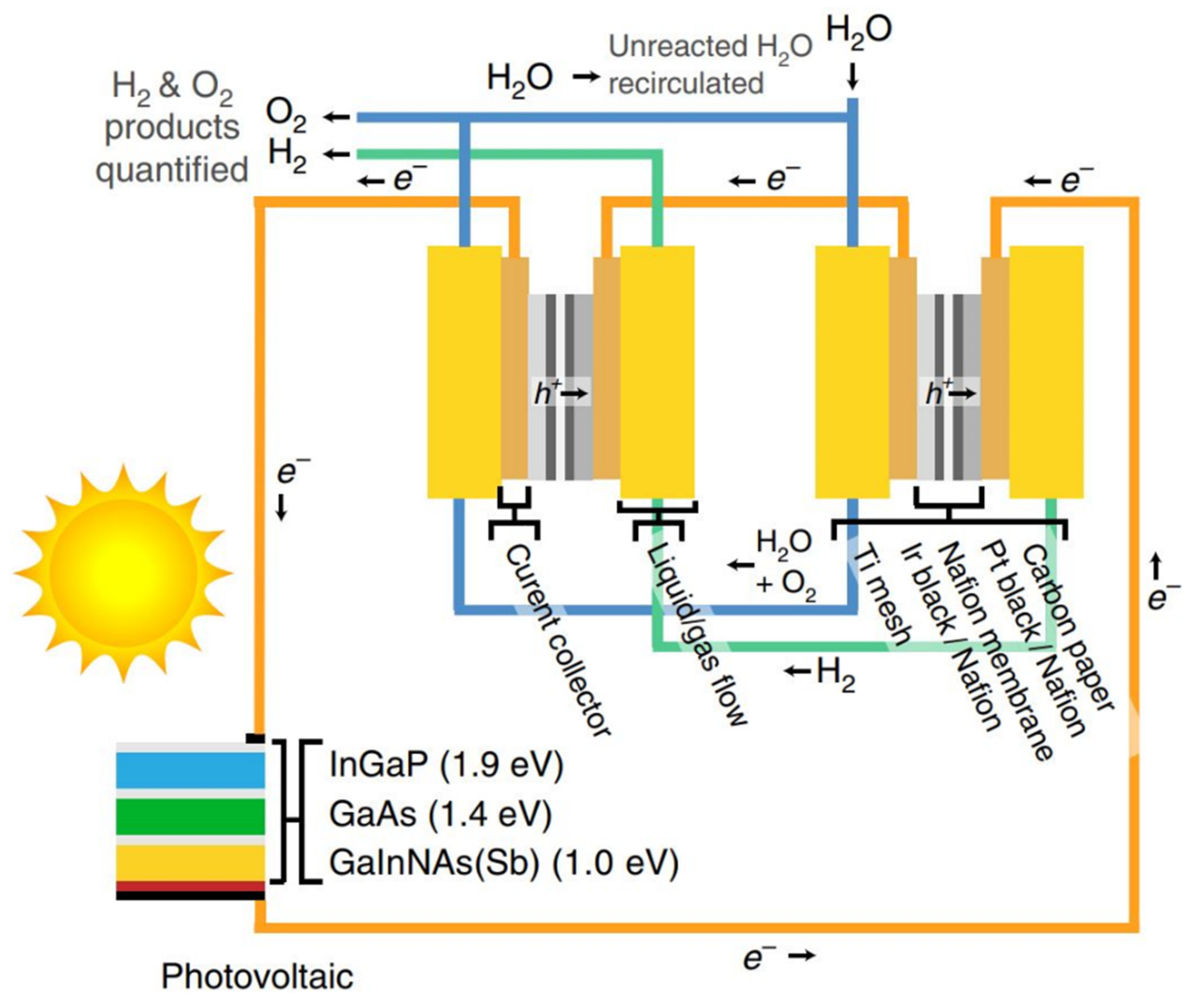 Figure 3. Schematic of PV-electrolysis system for hydrogen production [249].According to the techno-economic assessments in 2009, the cost of hydrogen production using the HyS process with nuclear reactor is $5.34–6.18 per kg [250]. On the other hand, the costs of solar-driven hydrogen production are approximately in the range of $2.64–7.58 per kg depending on the solar plant efficiency, heliostat cost, location, thermochemical efficiency, etc. [136,251,252]. It has been reported that the sulfur dioxide depolarized electrolyzer (SDE) can significantly reduce the costs and electrical power required for the process [134]. It should also be noted that the realistic thermochemical efficiency of the S-I cycle is in the range of 35–38% based on hydrogen LHV, and costs of hydrogen production with an S-I cycle equipped with a nuclear reactor is in the range of $3.50–12.0 per kg [253,254]. As listed in Table 1, D’Souza [189] estimated the overall cost of hydrogen production (per kg hydrogen) in different cycles in 2015 and 2025. US DOE chose these technologies in collaboration with the TIAX laboratory as the most promising thermochemical water-splitting cycles. As has been established by the US DOE, hydrogen production costs should be in the range of $6 and $2–3 per kg H2 in 2015 and 2025, respectively. As can be seen in this table, the ferrite cycle (Ni ferrite thin film) shows great potential for hydrogen production, followed by the HyS cycle. However, further developments are required to satisfy the long-term stability and efficiency of these cycles to decrease capital expenditures (CAPEX) and operating expenses (OPEX).Table 1. Hydrogen production costs ($ per kg H2) of different thermochemical water splitting cycles [189].
Figure 3. Schematic of PV-electrolysis system for hydrogen production [249].According to the techno-economic assessments in 2009, the cost of hydrogen production using the HyS process with nuclear reactor is $5.34–6.18 per kg [250]. On the other hand, the costs of solar-driven hydrogen production are approximately in the range of $2.64–7.58 per kg depending on the solar plant efficiency, heliostat cost, location, thermochemical efficiency, etc. [136,251,252]. It has been reported that the sulfur dioxide depolarized electrolyzer (SDE) can significantly reduce the costs and electrical power required for the process [134]. It should also be noted that the realistic thermochemical efficiency of the S-I cycle is in the range of 35–38% based on hydrogen LHV, and costs of hydrogen production with an S-I cycle equipped with a nuclear reactor is in the range of $3.50–12.0 per kg [253,254]. As listed in Table 1, D’Souza [189] estimated the overall cost of hydrogen production (per kg hydrogen) in different cycles in 2015 and 2025. US DOE chose these technologies in collaboration with the TIAX laboratory as the most promising thermochemical water-splitting cycles. As has been established by the US DOE, hydrogen production costs should be in the range of $6 and $2–3 per kg H2 in 2015 and 2025, respectively. As can be seen in this table, the ferrite cycle (Ni ferrite thin film) shows great potential for hydrogen production, followed by the HyS cycle. However, further developments are required to satisfy the long-term stability and efficiency of these cycles to decrease capital expenditures (CAPEX) and operating expenses (OPEX).Table 1. Hydrogen production costs ($ per kg H2) of different thermochemical water splitting cycles [189].Years HyS CuCl Ferrite SA ZnO MnO S-I 2015 5.68 6.83 4.06 7.78 6.07 - - 2025 3.85 5.39 2.42 4.71 4.18 4.63 4.68 Hydrogen production through thermochemical water-splitting cycles is a clean and attractive method for addressing the global hydrogen energy demand. Currently, the most common methods for hydrogen generation are fossil-based technologies, and they result in air pollution and greenhouse gas emissions, resulting in global warming. Clean hydrogen can be produced using thermochemical cycles, but these cycles need to be studied to improve efficiency and lower the overall operation costs. A number of the recent studies and their significant findings has been summarised in Table 2. As listed in this table, each technology has weaknesses and strengths compared to the other ones. Overall, it is suggested that future studies should focus on improving the overall efficiency of the cycles, especially the Cu-Cl low-temperature cycle as well as zinc oxide and ferrite high-temperature cycles, by using the most cost-effective catalysts, optimizing structural and microstructural properties, removing impurities from the cycles, promoting the redox reactions and conversion rates, doping, handling the byproducts, etc. Since catalysts can enhance reaction rates and help improve the hydrogen yield, a cost-effective catalyst selection will be helpful. Nanomaterials can also improve the efficiency of the cycles, but their weaknesses, such as agglomeration, grain growth, and costs, should be addressed efficiently. Non-stoichiometry can facilitate the reactions and promote hydrogen production in metal oxide cycles. The potent combination between the thermodynamics and kinetic properties of the reactions and the structural/microstructural properties of the materials such as structure, morphology, composition, etc. and possible new synthesis routes should be considered in the subsequent studies to improve the cost-effectiveness of the cycles further. Implementing simulations for predicting the results of using new materials, new synthesis conditions, rate of reactions in each step, etc., can be beneficial.Table 2. Summary of the significant discoveries of some of the essential thermochemical water-splitting cycles.Cycle Major Discovery Ref. S-I Optimization of the Bunsen section for liquid–liquid separation.
Increasing iodine content improved separation characteristics.
The optimum I2/H2SO4 ratio was in the range of 2.45–3.99 at 70–85 °C[107] S-I Developed a microporous membrane resistant to sulfur trioxide composed of α-alumina support, ZrO2-SiO2 intermediate layer, and organosilica sol top layer. High Si:Zr ratio and large pore of the ZrO2-SiO2 showed higher O2/SO3 selectivity and higher chemical stability against SO3 [115] S-I Developed a modified cycle with fewer steps and used a steam boiler. The modified cycle has a higher HI decomposition rate, and the Bunsen reaction happened at lower temperatures. [116] S-I Catalysts with hierarchical pore structure and higher specific surface area and micropore proportion of about 50% showed higher catalytic activity. [117] S-I N doping promotes HI decomposition rate [102] HyS Increasing Fe content in Fe/Al and Fe/Ti binary metal oxide catalysts improved catalytic activity [143] HyS Cr, Ce, U, Mn, and Ni form stable sulphates and are not suitable catalysts for sulfuric acid decomposition. t/BaSO4-TiO2, Pt/TiO2, Pt/ZrO2, and Pt/SiO2 are the most suitable catalysts for the HyS cycle. [135] HyS PtxPdy thin film deposited on a Si wafer showed high catalytic activity [146] HyS Increasing Ni dopant in PtxNiy/C catalysts increased electron vacancies and improved catalytic performance [148] HyS Incorporating ceria in Pt/C composite catalyst increased catalyst active area and improved its performance [150] Cu-Cl Developing a novel integrated system for producing nitrogen, methane, ammonia, oxygen, and carbon dioxide [168] Cu-Cl Optimizing the temperature of the hydrolysis step can minimize the number of byproducts. [172] Cu-Cl Cu-Cl is the most promising cycle for large-scale hydrogen production. [176] ZnO/Zn Partially reduced ZnO showed higher catalytic performance at elevated temperatures (R = 57.2%) [193] ZnO/Zn Hindered recombination and lowered the reaction temperature of the first step (methane or carbon as reducing agent) [197] ZnO/Zn A negative axial temperature gradient reduced the steam and inert gas proportion [199,202] SnO2/SnO Reducing O2 partial pressure to about 10−3 bar decreased thermal reduction temperature [209] Fe3O4/FeO Non-stoichiometric wustite have higher defect densities and showed higher reaction rates [188] Ferrite Zirconia support enhances the energy radiation absorption and lowers the temperature [186] Ferrite Ceramics nanoparticles can slightly increase hydrogen yield and hinder grain growth [219] Ferrite ZrO2 can reduce the high-temperature sintering and improve catalytic activity [218] Ferrite Calcium-stabilized zirconia forms calcium zirconate promotes high active phase dispersion and improves hydrogen yield [222] Ferrite Sacrificial (carbon black and PEG) and zirconia plates improved the thermal stability of Ni-ferrite samples [218] Ferrite Core-shell NiFe2O4/Y2O3 nanoparticles showed stable hydrogen volume, but lower rates of hydrogen production than that of mixed powders [219] Ferrite Hercynite formation in alumina and cobalt ferrite decreased ferrite reduction temperature [224]
This entry is adapted from the peer-reviewed paper 10.3390/en15031209
