β-glucosidases (EC. 3.2.1.21) are enzymes that hydrolyze glucosidic bonds of oligosaccharides, in special disaccharides, such as cellobiose, realizing glucose at the end of the process. They are highly used in second-generation biofuel production.
- enzymes
- bioinformatics
- β-glucosidase
- biofuel
- second-generation biofuel
1. Introduction
In second-generation biofuel production, they act in synergy with other classes of enzymes, such as endo-glucanases and exo-glucanases [2][3], to obtain fermentable sugars from biomass. However, the literature has described that most β-glucosidase enzymes are inhibited by glucose, which has been considered one of the most impacting bottlenecks for high-efficient industrial production [4].
Recently, a more efficient class of β-glucosidases have been described in the literature. They are called glucose-tolerant, due to their high resistance to inhibition even in high glucose concentrations [5]. This class of enzymes has excellent potential for use in industrial applications such as biofuel production. Hence, glucose-tolerant β-glucosidase enzymes have been an essential target of several studies to detect mutations that transform non-tolerant enzymes into glucose-tolerant enzymes [6].
Most glucose-tolerant β-glucosidases are obtained from glycoside-hydrolase family 1 (GH1). Proteins from this family have a conserved folding structure called TIM-barrel [7]. Figure 1 illustrates the three-dimensional structure of GH1 β-glucosidase from the fungus Humicola insolens complexed with a glucose molecule [8].
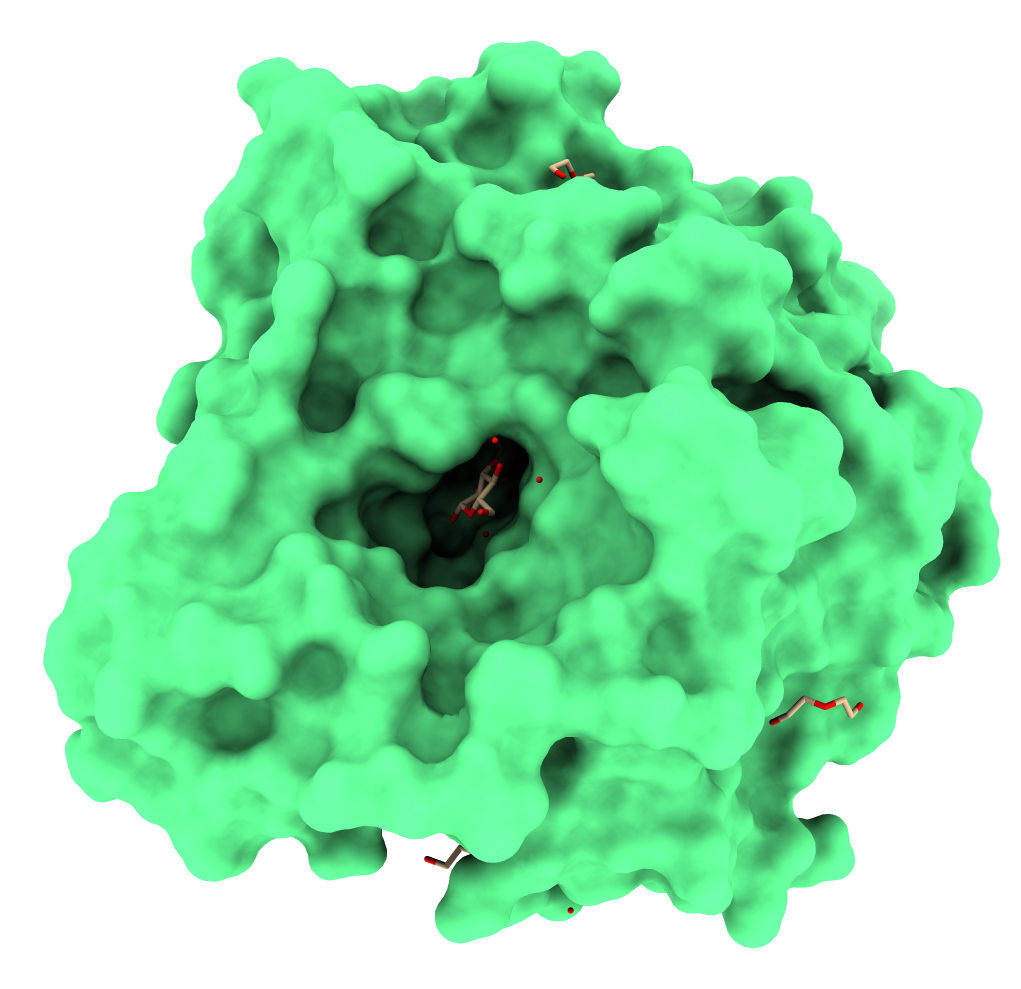 Figure 1. GH1 β-glucosidase from the fungus Humicola insolens. Obtained from PDB ID: 4MDP. Figure generated using ChimeraX [9].
Figure 1. GH1 β-glucosidase from the fungus Humicola insolens. Obtained from PDB ID: 4MDP. Figure generated using ChimeraX [9].
In conclusion, β-glucosidase enzymes have an excellent potential for second-generation biofuel production. However, genetic engineering applications are still necessary to improve the activity of non-tolerant enzymes. Additionally, bioinformatics applications have been successfully used to bring new insights to detect sites for mutations that could improve their activity.
2. Application
References
- James R. Ketudat Cairns; Asim Esen; β-Glucosidases. Cellular and Molecular Life Sciences 2010, 67, 3389-3405, 10.1007/s00018-010-0399-2.
- Leon Sulfierry Corrêa Costa; Diego César Batista Mariano; Rafael Eduardo Oliveira Rocha; Johannes Kraml; Carlos Henrique Da Silveira; Klaus Roman Liedl; Raquel Cardoso De Melo-Minardi; Leonardo Henrique Franca De Lima; Molecular Dynamics Gives New Insights into the Glucose Tolerance and Inhibition Mechanisms on β-Glucosidases.. Molecules 2019, 24, 3215, 10.3390/molecules24183215.
- Diego Mariano; Naiara Pantuza; Lucianna H. Santos; Rafael E. O. Rocha; Leonardo Lima; Lucas Bleicher; Raquel Cardoso De Melo-Minardi; Glutantβase: a database for improving the rational design of glucose-tolerant β-glucosidases. BMC Molecular and Cell Biology 2020, 21, 1-15, 10.1186/s12860-020-00293-y.
- Andreza P. Garbin; Nayara F.L. Garcia; Gabriela F. Cavalheiro; Maria Alice Silvestre; André Rodrigues; Marcelo F. DA Paz; Gustavo G. Fonseca; Rodrigo S.R. Leite; β-glucosidase from thermophilic fungus Thermoascus crustaceus: production and industrial potential. Anais da Academia Brasileira de Ciências 2020, 93, e20191349, 10.1590/0001-3765202120191349.
- Diego Mariano; C. Leite; L.H.S. Santos; L.F. Marins; K.S. Machado; Adriano Velasque Werhli; L.H.F. Lima; R.C. De Melo-Minardi; Characterization of glucose-tolerant β-glucosidases used in biofuel production under the bioinformatics perspective: a systematic review. Genetics and Molecular Research 2013, 16, 1, 10.4238/gmr16039740.
- Diego César Batista Mariano; Lucianna Helene Santos; Karina Dos Santos Machado; Adriano Velasque Werhli; Leonardo Henrique França De Lima; Raquel Cardoso De Melo-Minardi; A Computational Method to Propose Mutations in Enzymes Based on Structural Signature Variation (SSV). International Journal of Molecular Sciences 2019, 20, 333, 10.3390/ijms20020333.
- Nozomi Nagano; Christine A Orengo; Janet Thornton; One Fold with Many Functions: The Evolutionary Relationships between TIM Barrel Families Based on their Sequences, Structures and Functions. Journal of Molecular Biology 2002, 321, 741-765, 10.1016/s0022-2836(02)00649-6.
- Priscila Oliveira de Giuseppe; Tatiana De Arruda Campos Brasil Souza; Flavio Henrique Moreira Souza; Leticia Maria Zanphorlin; Carla Botelho Machado; Richard Ward; Joao Atilio Jorge; Rosa Dos Prazeres Melo Furriel; Mario Tyago Murakami; Structural basis for glucose tolerance in GH1 β-glucosidases. Acta Crystallographica Section D Biological Crystallography 2014, 70, 1631-1639, 10.1107/s1399004714006920.
- Eric F. Pettersen; Thomas D. Goddard; Conrad C. Huang; Elaine C. Meng; Gregory S. Couch; Tristan I. Croll; John H. Morris; Thomas E. Ferrin; UCSF ChimeraX : Structure visualization for researchers, educators, and developers. Protein Science 2020, 30, 70-82, 10.1002/pro.3943.
