Nanotechnologies have attracted increasing attention in their application in medicine, especially in the development of new drug delivery systems. With the help of nano-sized carriers, drugs can reach specific diseased areas, prolonging therapeutic efficacy while decreasing undesired side-effects. In addition, recent nanotechnological advances, such as surface stabilization and stimuli-responsive functionalization have also significantly improved the targeting capacity and therapeutic efficacy of the nanocarrier assisted drug delivery system.
- nanomedicine
- nanocarriers
- drug delivery
Note:All the information in this draft can be edited by authors. And the entry will be online only after authors edit and submit it.
1. Introduction
Nanotechnology has emerged to be an area of active investigation, especially in its applications in medicine [1]. The nanoscale manipulation allows optimal targeting and delivery as well as the controllable release of drugs or imaging agents [2]. Among all the applications of nanotechnology in medicine, nanocarrier assisted drug delivery system has attracted significant research interest due to its great translational value. The small size of the nanocarriers can help drugs overcome certain biological barriers to reach diseased areas [3,4]. Taking advantage of different nano-sized materials and various structures, nanocarriers can help poorly soluble drugs become more bioavailable and protect easily degraded therapeutics from degradation [5,6]. In addition, the modifiable surfaces of nanocarriers also expand their usability in different biomedical applications, especially in targeted therapy [7]. Indeed, their modification can not only stabilize but also functionalize them to be responsive to different stimuli, improving the therapeutic efficacy [7].
2. Strategies to Enhance Drug Delivery Efficiency
In nanomedicine, one common route for the carried-on drugs to reach the diseased area is to passively diffuse out of the nanocarriers that have accumulated in the diseased tissue [79]. As for adding targeting capacity to the nanocarriers, especially in cancer therapy, a common approach is surface addition of ligands that are specific to receptors overexpressed in certain cancer cells [55]. In an effort to develop smarter nanocarriers that can further improve the targeting efficiency and on-demand drug release, various stimuli-responsive nanocarriers are currently under development [9]. In the following sections, we will discuss the features and applications of some of the stimuli-responsive nanocarriers (Figure 2).
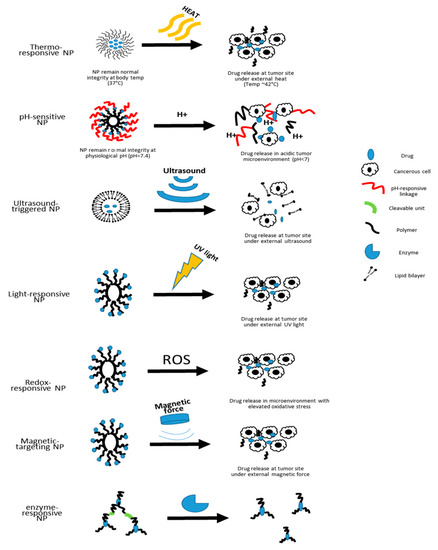
Figure 2. Schematic illustration of the drug-releasing mechanisms of different types of nanocarriers.
Currently, the stimuli-responsive nanocarriers can be activated by either exogenous stimuli, such as variations in temperature, magnetic field, ultrasound intensity, light or electric pulses, or endogenous stimuli, such as changes in pH, enzyme concentration or redox gradients [9]. The specific design of nanoscale stimuli-responsive systems enables the controlled drug biodistribution in response to specific exogenous or endogenous stimuli [80]. Through the stimuli responsiveness, on-demand drug release could be achievable [80,81,82].
2.1. Thermo-Responsive
Thermo-responsive nanocarriers are among the most investigated nanocarriers in cancer therapy for solid tumors [80]. The working concept is that thermo-responsive nanocarriers are able to retain their payload at 37 °C, the physiological body temperature, but rapidly release their payload at the heated tumor area (~40 to 42 °C) [80,83]. As nanotechnology advances, polymeric nanocarriers that exhibit lower critical solution temperature (LCST) and upper critical solution temperature (UCST) can have better control of drug release [84]. This strategy is helpful in the treatment of solid tumors as drugs can be loaded at 37 °C, and depending on the LCST or UCST, drugs can then be released by either cooling or heating the tumor after injection [84]. Based on the existing thermo-responsive technology, an additional pulsatile drug delivery system has been introduced by embedding thermo-responsive polymers to liquid crystals to achieve programmed drug release in concordance with the circadian rhythm [85].
2.2. pH-Responsive
As pH values vary in different biological compartments, pH-responsive nanocarriers will allow better control of site-specific drug release [86]. There are two main strategies that exist for the development of pH-sensitive nanocarriers. One is using polymers functionalized with ionizable groups that can undergo a conformational change upon encountering environmental pH change, and the other is the incorporation of acid-sensitive bonds that break in an acidic environment for drug release [80,87]. Due to the acidic microenvironment of tumor sites, pH-responsive nanocarriers rise to be a useful strategy for targeted cancer therapy [88]. The intracellular pH values also differ from the ones in the extracellular matrix [89].
A wide range of nanocarriers can be equipped with pH-responsivity [90]. For example, vaccines for hepatitis B virus are under development using pH-responsive liposomes to achieve cytosolic drug release [91]. Polymeric micelles that are functionalized to be pH-responsive are under active investigation for their application in chemotherapy [92,93]. To overcome certain disadvantages of some pH-sensitive polymers, such as uncontrolled drug-loading or drug-releasing rate as well as undesired toxicity, non-polymer pH-sensitive carbon dots were also developed for cancer therapy [94]. In addition, recently surface modified pH-responsive SWCTs have also been developed to co-deliver anti-cancer drugs and genes [95].
2.3. Ultrasound-Triggered
The ultrasound-triggered drug-releasing approach is an appealing on-demand drug-releasing strategy because of their non-invasiveness as well as the controllable frequency and duration in order to regulate the depth of tissue penetration [80]. Ultrasound generated mechanical forces can transform nanodroplets (such as liposomes) to nano-bubbles [80]. These ultrasound-genic nanobubbles increase the efficiency of delivering payloads to neighboring cells [80]. Prabhakar et al. have recently developed a nanobubble liposome complex that can be ultrasound triggered to deliver both imaging agents and anti-cancer drugs, suggesting a promising future for the theranostic application of ultrasound-triggered nanocarriers [96]. However, despite the fact that ultrasound is non-invasive, the frequency applied in order to trigger drug release differs from conventional settings for clinical imaging purposes. Such differences raise the concern of potential mechanical induced cell damage [97]. Another limitation is that the ultrasound beam may be attenuated by the hard tissues and certain tissue associated movements [98].
2.4. Light-Responsive
Another non-invasive and controllable approach for drug release is to incorporate light-responsive materials in the nanocarriers. Under certain wavelengths of light, these nanocarriers can either disassemble for drug release or shrink in size for deeper tissue penetration [80]. Wang et al. developed a near-infrared (NIR) light-responsive polymeric nanocarrier by incorporating selenium that can rapidly dissociate within minutes post NIR light exposure due to reactive oxygen species (ROS)-mediated selenium oxidation [99]. Such irreversible dissociation of nanocarriers promotes continuous drug release [99]. With the help of light-responsive nanomaterials, a high degree of spatiotemporal precision can be achieved, but the safety of light-responsive nanocarriers are still not well defined [100,101]. Specifically, the irreversible change of these nanocarriers raises concerns for the safety of byproducts [100].
2.5. Redox-Responsive
Given that oxidative stress has been found to be elevated in the pathogenesis of many diseases, another type of stimuli-responsive nanocarriers that have attracted significant interest are the redox-responsive nanocarriers [102,103,104]. The tumor microenvironment has certain features that are different from healthy tissues. For example, there is a significant elevation in the concentrations of glutathione and ROS in tumor microenvironments than in normal tissues [105]. With the help of redox-responsive polymers, these nanocarriers can significantly increase the concentration of drugs released in the diseased area [105,106].
Similarly, in diabetes, hyperglycemia induces cellular hypoxia through mitochondrial ROS production [106,107]. In order to benefit diabetic patients, Gu’s group has been advancing the painless microneedle patch using hypoxia-responsive nanoparticles that release insulin in a hypoxemic microenvironment. They first developed microneedle-array patches loaded with hypoxia-sensitive vesicles to provide fast glucose-responsive insulin delivery [108]. Subsequently, they improved their nanoparticles using H2O2-responsive polymeric vesicles integrated with transcutaneous patches for glucose-mediated insulin delivery [109]. Combining both technologies, they developed a new nanovesicle that is responsive to both hypoxic environment and H2O2 in order to enhance insulin delivery [110].
As ROS and redox signaling also play important roles in ischemia/reperfusion (I/R) injury, redox-responsive nanocarriers delivering anti-oxidant to the injured site could also potentially ameliorate I/R injury in the tissues [111,112,113]. For this purpose, Kang and colleagues have developed ROS-responsive nanocarriers for their application in various I/R injuries [114,115,116,117,118]. Recently, Elkassih et al. also developed degradable redox-responsive disulfide cross-linked nanogel drug carriers to decrease cytotoxicity and to increase drug uptake in areas with increased oxidative stress [119].
2.6. Magnetic Targeting
Nanocarriers can also be modified to be responsive to magnetic force. In this approach, the therapeutic agents are attached to or encapsulated in magnetic nanocarriers, which are often made of functionalized polymers [120]. Among all the candidate nanomaterials, the biocompatible superparamagnetic iron oxide nanoparticles (SPIONs) with modifications are the most widely used as part of the magnetic nanocarriers [121]. These nanocarriers are then injected into the bloodstream near the target site [120]. When the magnetic fields are applied over the target site, the magnetic force will drive the accumulation and release of the payloads [120]. Previous studies have shown that magnetophoresis can enhance the accumulation and penetration of nanocarriers into solid tumors [122]. However, the clinical translation of this approach is difficult due to its low efficacy and uncontrollable magnetic nanoparticle distribution [123,124]. One of the biggest challenges with the use of magnetic fields is that because the magnetic force falls off significantly with distance, the target sites are limited to the near-surface of the body [120]. Some groups attempted to overcome this challenge by implanting magnets within the body in order to reach deep tissue penetration [120]. However, the use of permanent magnets itself has become a limitation for the clinical translation of this approach [125,126]. In addition, the lack of real-time imaging and the difficulties in controlling magnetic force for precise delivery are also factors that limit its clinical use [125,126]. Using the oppositely polarized magnets, Liu et al. aimed to improve the weak magnetic force in older generations of magnetophoresis that can only be used to treat superficial tumors [122]. This method increased the penetration by five-fold and accumulation by three-fold of magnetic nanoparticles within solid tumors compared to passive enhanced permeability and retention effect [122].
2.7. Enzyme-Responsive Nanocarriers
In addition to the environmental stimuli such as pH and oxidative stress, enzymes that present within the cellular system can also be utilized as a trigger for targeted drug release [127]. Nanocarriers such as polymeric micelles, liposomes, and dendrimers are often functionalized by attaching cleavable peptides to the surface that are tailored to specific enzymes present in the targeting tissues [128,129,130,131]. In order to achieve the goal of targeted cargo delivery, several releasing mechanisms can be employed. In functionalized enzyme-responsive liposomes, the enzymes can either directly perturb the lipid bilayer structure, or cleave a lipopeptide or lipopolymer incorporated in the bilayer to achieve destabilization of the nanocarrier [129]. In addition, targeted enzymes can also remove the shielding polymers from the surface to increase cellular uptake, or activate a prodrug in the nanocarriers [129].
Based on the purpose of the treatment, a variety of enzymes can be utilized to trigger local drug delivery. For example, one unique feature of the cancerous tissues is the overexpression of a type of extracellular proteolytic enzyme, called the matrix metalloproteinases (MMPs) [132]. Based on this feature, several MMP-responsive nanocarriers have been developed as one approach for the targeted cancer treatment [133]. For targeted anti-inflammatory treatment, a protease secreted by neutrophils, called human neutrophil elastase (HNE) is exploited as a biological cue for controlled drug release from nanocarriers equipped with HNE-sensitive peptide linkers [134,135]. This approach can significantly increase the sensitivity of targeted anti-inflammatory treatment, as neutrophils are the first cells recruited to inflammatory sites [136]. Enzyme-responsive nanocarriers can also be applied to regulate coagulation locally. For this purpose, Bhat et al. developed a thrombin-responsive mesoporous silica nanoparticle (MSN) that is loaded with an anticoagulant drug and capped with a peptide containing a thrombin-specific cleavage site [137]. When the coagulation cascade is triggered, active thrombin can degrade the capping peptide sequence on the nanocarrier and release the anticoagulant locally [137]. Due to the site-specificity of thrombin, thrombin-responsive nanocarriers possess the advantage of spatiotemporal specificity in anti-thrombotic drug delivery [138].
While significant progress has been made in the development of enzyme-responsive nanocarriers, several challenges remain to be addressed for the wider application of this approach. For example, there are various enzyme subtypes existing in the biological system that share similar cleavage sites [130]. In addition, current imaging technologies are yet to satisfy the need for the confirmation of controlled drug release at the targeted areas [130].
2.8. Multimodal Nanocarriers
Despite the fact that stimuli-responsive nanocarriers have already significantly improved targeted drug delivery, they still face certain limitations. For example, stimuli-responsive nanocarriers utilizing external factors, such as ultrasound, light, heat, and magnetic forces, are limited to targets with known target localization. In addition, human physiology is a complex system. Nanocarriers that are responsive to pH and oxidative stress might potentially release cargoes at areas other than the diseased area because those non-targeted areas also share similarly elevated pH or oxidative stress, due to conditions such as metabolic acidosis, vascular occlusion, or other inflammations. By combining different stimuli-responsive properties, multimodal nanocarriers can improve the efficacy of nanomedicine, especially in cancer therapy [139]. Recently, nanocarriers that possess both thermo- and pH-responsiveness are being developed to treat certain cancers [140]. Using this dual stimuli-responsive system, Hiruta et al. developed a polymeric micelle for anti-cancer drug delivery that can be selectively up-taken with external thermal stimulation and effectively release its cargo at endosomal pH [141]. As each stimuli-responsive nanocarrier has different advantages and limitations (Table 2), with additional stimuli responsiveness, nanocarriers can be more attuned to the stimuli changes and exert therapeutic effects in a more precise fashion. Recently, a programmable polymer library for stimuli-responsive nanocarriers containing logic gates has also been developed to gather systemic information in order to achieve precision medicine [142,143].
Table 2. Summary of different stimuli-responsive nanocarriers.
| Stimuli Type | Advantages | Challenges/Limitations | References |
|---|---|---|---|
| Thermo-Responsive |
|
|
[80,83,84] |
| pH-Sensitive |
|
|
[80,86,87,88,90,91,92,93,94,95] |
| Ultrasound-Triggered |
|
|
[80,96,97,98] |
| Light-Responsive |
|
|
[99,100,101] |
| Redox-Responsive |
|
|
[103,105,106] |
| Magnetic-Targeting |
|
|
[122,123,124,125,126] |
| Enzyme-Responsive |
|
|
[127,128,129,130,131,133] |
Another example in the anticancer drug delivery system is the nanocarriers designed to respond to the tumor microenvironment which can switch its size and morphology in response to the acidic tumor microenvironment and near-infrared laser irradiation [144]. Jia et al. have recently developed a smart nanodrug that can switch its size and morphology in response to the acidic tumor microenvironment and near-infrared laser irradiation to effectively ablate a tumor, inhibiting tumor metastasis [144]. This nanodrug is assembled by a cytolytic peptide, an NIR-absorbing molecule, and a tumor-targeting polymer [144]. Under normal physiological environment, the assembly is a negatively charged nanosphere about 50 nm in size [144]. The acidic tumor microenvironment triggers the transformation of the nanodrug into net-like nanofibers [144]. The net-like structure helps to limit the mobility of tumor cells and also prolongs the drug retention time [144]. During photothermal therapy, the nanocomplex can be photodegraded into smaller nanospheres about 25 nm in size to allow deeper tumor penetration of the drug [144].
When combining gene and photothermal therapy, synergistic therapeutic effects have been observed, suggesting the advantages of multimodal nanomedicine [145]. In a similar effort for on-demand drug release, Deng et al. developed a new liposomal drug delivery platform that can control payload release only when triggered by x-ray radiation [146]. This liposome incorporates gold nanoparticles with a photosensitizer called verteporfin [146]. Under radiation, the photosensitizer produces singlet oxygen to destabilize the liposomal membrane, allowing payload to be released from the liposome, while the gold nanoparticles are used for radiation enhancement [146]. This platform could provide synergistic therapeutic effects in chemotherapy when combined with radiotherapy [146]. The main drawback of this platform design, however, is that the photosensitizer, as well as the gold nanoparticle incorporated, generate a certain level of ROS in the tissues that could be damaging [146].
Continued efforts are being made to advance theranostic nanocarriers. By functionalizing the surface of FDA-approved iron oxide nanoparticles with an imaging contrast agent and a peptide activatable by a tumor-specific enzyme new theranostic nanocarriers can achieve enzyme-specific drug delivery at the site of the tumor and simultaneous MRI imaging [147]. Conjugated polymer nanosystems are developed to combine diagnostic imaging together with photothermal therapy and drug delivery in cancer therapy [148]. Li et al. have also functionalized the carbon quantum dots so that they structurally mimic large amino acids that can selectively accumulate at tumor sites for both imaging and drug delivery purposes [149]. A recent review by Riccardi et al. summarizes the development of different nanocarriers with various decorations (or functionalization) for the improvement in bioavailability, pharmacokinetics, and specificity of anticancer ruthenium-based drugs [150].
2.9. Bioinspired Nanocarriers
Besides using biodegradable and biocompatible synthetic materials for the development of nanocarriers, other bioinspired natural materials are also being explored for their application in drug delivery systems. One strategy is to develop biomimetic nanoparticles by using the cell membrane as camouflage [151]. For example, utilizing the concept of biomimetic functionalization of the nanocarriers, Liu et al. integrated a red blood cell (RBC) membrane vesicle with near-infrared persistent luminescence nanophosphors to ensure the nanocarriers can bypass macrophage uptake and systemic clearance to improve circulation time for bio-imaging and drug delivery [152]. Santos’ group further engineered the isolated RBC membranes to form nanoerythrosomes (NERs), i.e., derivatives of RBCs with an average diameter of 100 nm, for drug delivery [153]. Similarly, using a cancer membrane as camouflage, a tumor homing nanocarrier that carries imaging and/or therapeutic moieties can also provide a new platform for targeted drug delivery [154,155]. Compared to RBC membranes, cancer cell membranes alone are unstable and have insufficient drug entrapment, thus cannot act as an autonomous drug delivery system without the support of other nanomaterials [153]. Therefore, Balasubramanian et al. combined cancer cell membrane material with porous silicon nanoparticles to develop nanocarriers that serve as artificial organelles in order to supplement cellular functions under oxidative stress [156].
In addition to using bioinspired nanomaterials to develop new nanocarriers, existing nanocarriers can also be modified in a bioinspired fashion. Zhang et al. recently developed a liposomal nanocarrier with a modified surface that has a short nontoxic peptide derived from Aβ1-42 that specifically interacts with the lipid-binding domain of apolipoproteins [157]. These nanocarriers absorb plasma apolipoproteins A1, E, and J, resulting in the exposure of the receptor-binding domain of apolipoproteins to achieve brain-targeting drug delivery [157].
3. Strategies to Enhance Therapeutic Efficacy Using Different Payloads
3.1. Cell Replacement
Nanocarriers can also deliver other payloads such as cells to the diseased area. Such an approach can be applied in cell replacement therapies. For example, for type I diabetic patients, islet transplantation is a promising treatment [158]. However, it is limited by the shortage of donors and the significant side effect of immunosuppression [158]. In order to overcome this challenge, recent advancements in nanotechnology enables the encapsulation of the islet in immune-isolating membranes with chemical modifications for transplantation [29,158,159]. Using mesenchymal stem cells (MSCs) as cell-based drug delivery vectors for tumor-homing cancer treatment has also shown some promising results [160]. However, the broad biodistribution of MSCs also raises concerns for toxicity to non-target peripheral tissues [160]. A wider application of nanocarrier assisted cell replacement therapy still requires more investigation.
3.2. Gene Therapy
Another area of investigation that is currently on the horizon is the concept of nanocarrier assisted gene therapy. One important strategy in gene therapy is the use of small interfering RNA (siRNA) to silence disease-causing genes [161]. Currently, there are more than 20 siRNA based therapies in clinical trials [161]. However, there are two main concerns of RNA interference (RNAi) potency and specificity [162]. Transporting siRNA across the cell membrane is challenging due to its anionic property [163]. In addition, naked siRNA has immunostimulatory effects and is easily degraded in the bloodstream [163]. Viral vectors have long been used to deliver siRNA, however, they have drawbacks, such as being immunogenic and cytotoxic [164]. Being non-viral and equipped with targeting capacity, nanocarriers have drastically helped the emergence of RNAi therapeutics [161]. Different nanocarriers are being applied in the delivery of siRNA, such as nucleotides, lipids, and polymers [161,163]. The biocompatibility and design flexibility of nanocarriers allow better control of siRNA delivery to achieve desired gene knockdown efficiency [162,163,164].
Among all the nanocarriers under development for their potential to assist in gene delivery, lipid nanocarriers have shown the most promising results in the clinical translation of siRNA therapy [165,166,167,168,169]. Lipid nanoparticles can protect siRNA from degradation, and facilitate endocytosis and endosomal escape [168]. The first nanoparticle assisted targeted RNAi delivery in humans was reported in 2009 [170]. In this study, the nanocarriers were designed to passively accumulate and permeate in solid tumors [170]. With the help of CRISPR-Cas9 technology, new lipid nanocarriers capable of selective organ targeting (SORT) have been developed [171]. These nanocarriers can target extrahepatic tissues with the aid of targeting molecules for selective organs, revolutionizing tissue-specific gene editing [171]. Lipid nanocarrier assisted nucleic acid delivery is also under active investigation for its potential use in the development of prophylactic vaccines [172]. In addition, recently another type of nanocarrier similar to the liposome, called noisome, has also been developed [173]. Niosomes are self-assembled vesicles made up of single-chain non-ionic surfactants combined with appropriate amounts of cholesterol or other lipids [173]. Similar to liposomes, niosomes are capable of carrying hydrophilic or lipophilic drugs but are more stable, less expensive, and easier to manipulate [173]. They have the potential to be an alternative gene delivery system.
4. Conclusions
In this review, we discussed different types of nanocarriers applied in the drug delivery system. The use of nanocarriers, whether with single-featured nanomaterials and/or structures or in a hybrid fashion, has largely expanded the platform for drug delivery. In addition, the functionalization of nanocarriers that enables them to be sensitive to different stimuli (such as pH, heat, light, or oxidative stress) has further broadened their capacities for the delivery of different therapeutics. While tremendous advancements have been achieved in this field over the years, challenges remain to overcome in order to improve the translational value of current nanomedicine research. Specifically, the long-term safety profiles of these nanocarriers need to be carefully evaluated. Looking ahead, the nanocarrier assisted-delivery system holds great potential in improving therapeutic efficacy.
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics12090837
