Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Microbiology
Sepsis is a life-threatening condition that arises when the body’s response to an infection injures its own tissues and organs. Despite significant morbidity and mortality throughout the world, its pathogenesis and mechanisms are not clearly understood.
- sepsis
- pathogenesis
- management
- diagnosis
- antibiotic therapy
1. Introduction
Sepsis is an important syndrome associated with significant morbidity and mortality. The true extent of sepsis is not fully understood due to its variability and the lack of specific epidemiological data, due to different diagnostic criteria and definitions. The World Health Organization (WHO) has stated that the worldwide annual mortality due to sepsis is around 6 million, with most of these deaths being preventable [1,2,3,4,5,6,7]. Originally defined as “the decomposition of animal or vegetable matter in the presence of bacteria” [8], “sepsis” is currently identified as “a life-threatening condition that arises when the body’s response to an infection injures its own tissues and organs” [9]. Initiated by an invading pathogen, generally represented by bacteria and, less frequently, by viruses or fungi, sepsis results in an inflammatory process in which the body’s own response has a deleterious effect upon itself. This pathophysiological response can culminate in multiorgan failure, usually due to a combination of cardiovascular, cellular, coagulation and endothelial dysfunction [10], eventually leading to septic shock, a clinical proinflammatory response, predominantly cytokine-mediated (see Figure 1).
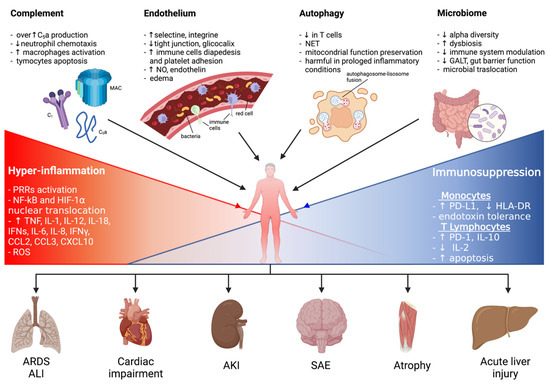
Figure 1. A graphical summary of the pathophysiological mechanisms involved in sepsis onset and persistence and related organ injuries. ALI: acute lung injury; AKI: acute kidney injury; ARDS: acute respiratory distress syndrome; CCL: C-C motif chemokine ligand; CXCL: C-X-C motif chemokine ligand; ET: endotoxin tolerance; GALT: gut-associated lymphoid tissue; HIF-1α: hypoxia-inducible factor-1α; HLA-DR: human leukocyte antigen-DR isotype; IFNγ: interferon γ; IL: interleukin; LPS: lipopolysaccharide; MAC: membrane attack complex; NET: neutrophil extracellular trap; NF-kB: nuclear factor kappa-light-chain-enhancer of activated B cells; NO: nitric oxide; PD-1: programmed cell death 1 receptor; PD-L1: programmed cell death ligand 1; PRRs: pattern recognition receptors; ROS: reactive oxygen species; SAE: sepsis-associated encephalopathy.
2. Pathophysiology of Sepsis
2.1. The Innate Immunity
The mechanisms resulting in the development of sepsis are very complex and not completely understood. However, it is well established that at the beginning of sepsis the inflammatory response is mediated by the activation of the innate immune system cells, mainly represented by macrophages, monocytes, neutrophils, and natural killer cells. Multiple infection-derived microbial products are simultaneously recognized by complement and specific cell-surface receptors. Amongst these, toll-like receptors (TLRs) are transmembrane receptors expressed by monocytes and macrophages and able to detect extracellular pathogen-associated molecular patterns (PAMPs) (such as bacterial endotoxins and fungal β-glucans) and damage-associated molecular patterns (DAMPs) released from injured endogenous cells (such as ATP, high mobility group proteins, and mitochondrial DNA). To this extent, nod-like receptors (NODs), which are expressed intracellularly, recognize pathogens invading the cytosol. Furthermore, retinoic acid inducible gene (RIG)-like receptors, mannose-binding lectin (MBL), and scavenger receptors also take part in this process [11].
Therefore, the binding between cellular receptors and different components of bacteria, viruses, and fungi, as well as host products derived from tissue damage, induces multiple intracellular signaling pathways, ultimately leading to the expression of several common gene classes involved in inflammation, adaptive immunity, and cellular metabolism, the second key step in the activation of the immune response during sepsis.
3.2. The Complement System
The complement system, which consists of multiple proteins in body fluids, receptors, and regulatory proteins, carries out a defensive action against infectious agents and acts as an immune sensor, effector, and regulator. Complement activation can be initiated via three different pathways: the classical (including antibodies, C1q, C2, and C4), the alternative (including complement factor B and spontaneous C3 hydrolysis to form C3b), and the lectin pathway (including MBL and ficolins) [14,15]. The common result of these pathways is the cleavage of C3 and C5 to generate anaphylatoxin peptides (i.e., C3a and C5a), C3b, and C5b. C3b is an important phagocytosis-promoting product, whereas C5b interacts with C6–C9 to form the membrane attack complex on cell membranes. C5a, under conditions of regulated production, supplies defensive functions by enhancing chemotactic responses of neutrophils, phagocytosis, and oxidative burst that is involved in killing bacteria [16,17,18].
The role of complement in sepsis pathogenesis might appear ambiguous. On one hand, C3 deficiency, which results in the inhibition of most complement effector functions, clearly increases sepsis-associated mortality in animals [19]; these observations underline the pivotal role of complement as a defense mechanism against invading microbes. Contrariwise, other data have indicated that inhibition of C5a signaling improves the survival of experimental animal models [20,21]. Increased production of C5a, as occurs during sepsis, can lead to adverse systemic consequences. Neutrophils become functionally paralyzed [22], unable to respond chemotactically to C5a, but also to the chemotactic peptide N-formyl-Met-Leu-Phe (fMLP), which is produced by bacteria [23].
3.3. The Role of the Endothelium
Sepsis is not only a state of systemic inflammation, but also a state of deregulated hemostasis. Hemostasis is a complex process regulated by the endothelium, soluble plasma molecules, platelets, and leukocytes; it not only is involved in the balance between pro- and anticoagulant forces, but also directs platelet and fibrin clotting to areas of focal vascular injury [28]. Sustained inflammation during severe sepsis drives hemostasis in a condition of deregulation characterized by a prothrombotic and antifibrinolytic state, organ ischemia, and multiple organ dysfunction syndrome.
Sepsis is associated with severe endothelium dysfunction leading to deregulation of vascular reactivity and hemostasis. This damage of the endothelial cells (ECs) is considered pivotal to the progression to organ failure during sepsis. Under normal conditions, the endothelium serves as an anticoagulant surface that regulates the flow of gases, water, solutes, lipids, proteins, and other macromolecules within the microcirculation [29,30]. The endothelium integrity is maintained by the cell cytoskeleton (actin), intercellular adhesion molecules (tight junctions), and numerous supportive proteins. During sepsis, these structures are broken up essentially in response to neutrophil and platelet adhesion, the release of inflammatory mediators, and toxic intermediates.
3.4. Autophagy
Autophagy is a highly conserved degradative pathway involved in maintaining intracellular homeostasis under physiological conditions, playing a crucial role in the pathogenesis of inflammation and infectious diseases [37,38]. There are three types of autophagy: macroautophagy, chaperone-mediated autophagy, and microautophagy. To eliminate damaged proteins and organelles, as well as cytoplasmatic bacteria and pathogens [39], cells exploit this adaptive mechanism to protect themselves from damages and apoptosis [40]. Several intracellular signaling pathways are responsible for autophagy induction, such as 5′ adenosine monophosphate-activated protein kinase (AMPK) and c-Jun N-terminal kinase (JNK)/p38, two MAPK pathways, generating ROS and regulating the NF-κB under activation of TLR4 and TLR9, respectively [41,42]. Recently, the induction of autophagy has received increased attention in the context of sepsis: it mainly protects the host against multiorgan dysfunction syndrome (MODS) by preventing immune cell apoptosis, maintaining the homeostatic balance between pro- and anti-inflammatory cytokines, and preserving mitochondrial functions [43,44,45,46].
3.5. Downregulation of the Immune System
Besides the systemic inflammatory response characterizing sepsis during the early stages of the process, a prolonged state of immunosuppression also occurs in both the initial and late phases of the disease [62,63]. Indeed, patients who survive the early inflammatory stage of sepsis enter a late phase characterized by profound immunosuppression: these patients frequently experience ongoing infectious foci, despite antimicrobial therapy; reactivation of latent viral infection; and acquisition of secondary hospital-acquired infections, often with opportunistic microorganisms, which usually do not tend to infect patients with normal immune status.
Sepsis has been described as a two-phase process where an initial hyperinflammatory phase is followed by a prolonged immunosuppressive phase [64,65]. However, in clinical practice, it is evident that these two phases tend to overlap. Indeed, several studies have shown that both proinflammatory and anti-inflammatory responses occur simultaneously in the first stage of sepsis [66,67,68]. The net effect of such mechanisms results in immunosuppression involving both the innate and the adaptive immune systems.
3.6. The Role of the Microbiome
The microbiome is the microbic consortium of bacteria, viruses, fungi, and protozoa living upon (skin) and inside (gut, lung) our body. The last few decades have seen an increasing interest regarding the physiological and pathogenetic role of the microbiome in several medical conditions, included sepsis. Indeed, the gut microbiome exerts numerous physiological roles as an independent organ within the human body: it produces functionally active metabolites which influence immune functions of several immune cells; it increases the gut barrier function, inhibiting hematic translocations of resident microorganisms; and it directly promotes the maturation of local immune cells, driving the expansion of antigen-specific activated T cells and enhancing responsiveness of immune cells to cytokines [89].
3.7. Cellular, Tissue, and Organ Failure
Sepsis is also described as a systemic disorder, affecting all organs of the body. Although the molecular basis of organ failure remains unclear, six types of organ dysfunction predominantly characterize sepsis: neurological (altered mental status), pulmonary (hypoxemia), cardiovascular (shock), renal (oliguria and/or increased creatinine concentration), hematological (decreased platelet count), and hepatic (hyperbilirubinemia). The underlying mechanism behind tissue and organ dysfunction in sepsis seems to be a diminished oxygen delivery to and utilization by cells with a consequent increased anaerobic glycolysis and lactic acid production. Several factors, including hypotension, reduced red-cell deformability, and microvascular thrombosis, contribute to impair tissue oxygenation in septic shock in addition to mitochondrial damage caused by oxidative stress [101]. All these mechanisms in conjunction with systemic hyperinflammation and sustained immunosuppression, generalized increased catabolism, insulin resistance, and hyperglycemia can contribute to the cellular level damage.
4. Diagnosis of Sepsis
4.1. MDR and Sepsis
In the last few decades, a specific sepsis population with a high mortality risk is accounted for by patients with septic shock by multidrug-resistant (MDR) microorganisms, with Gram-negative pathogens being responsible for most cases [102]. In particular, an increased frequency of MDR Gram-negative pathogens, such as MDR Acinetobacter baumannii (MDR-AB) and Klebsiella pneumoniae carbapenemase-producing Klebsiella pneumoniae (KPC-Kp), have been observed among critically ill intensive care unit (ICU) patients.
Although several risk factors for MDR microorganism infections have been identified (see Table 1), the real causes for this increased risk are still unclear, and the appropriateness of initial antibiotic therapy still represents a crucial variable in septic patients, thus affecting the clinical outcome [105,106,107,108].
Table 1. Main risk factors for MDR infections.
| Advanced age |
| Diabetes |
| End-stage liver disease |
| Immunosuppressive therapy |
| Use of corticosteroids |
| Malignancy |
| Organ transplantation |
| Recent surgery |
| Recent exposure (<3 months) to antibiotic therapy |
| Prior hospital admission |
| MDR colonization |
| Local epidemiology |
Legend. MDR: multidrug-resistant.
4.2. Diagnostic Tools
The ideal diagnostic technology for sepsis should include the following characteristics: a rapid and broad-based detection, minimal invasiveness, clinical sample usage with low specimen volumes, high sensitivity and specificity for the immediate initiation of targeted antibiotic use in the presence of signs and symptoms of systemic inflammation, and detection of drug resistance and unknown and emerging pathogens. So far, blood culture is commonly known as the gold standard for the detection of microbial pathogens in the bloodstream. However, the organisms’ growth to detectable levels in routine blood cultures can take up to 5 days, with additional time being required for identification (24 h) and testing for antibiotic susceptibility (48 h) [111,112,113,114]. False positives via contamination during sample collection (e.g., Staphylococcus epidermidis) are also common. Moreover, adults with bacteremia and/or with fungemia might receive an inappropriate treatment before microbiology culture results become available [115].
5. Therapeutic Approach
5.1. General Considerations
Besides supportive therapy (vasopressor administration, mechanical ventilation, renal replacement therapy), the treatment of sepsis and septic shock is based on empirical antibiotic therapy and infection source control. The 2021 Surviving Sepsis Campaign provides recommendations about the management of sepsis and septic shock [136]. As reported above, it is crucial to collect blood cultures prior to antibiotic administration and to start therapy with broad-spectrum antibiotics within 1 h after recognition of sepsis or septic shock condition. In 2014, the MEDUSA trial showed that delay in antimicrobial therapy and source control was associated with increased mortality in sepsis and septic shock patients: every hour the antibiotic therapy was delayed, mortality increased by 2% [137,138].
Nevertheless, it is important to administer appropriate antibiotic therapy rather than early administration of any antibiotic.
5.2. Patients in ICU
Critically ill patients with severe sepsis present a significant fluid shift from the intravascular compartment to interstitial space, caused by aggressive fluid resuscitation and hyperdynamic state associated with sepsis itself [142,143,144,145,146,147,148,149,150,151,152,153,154]. Extracellular fluid changes may also be enhanced by edematous states, pleural effusion, postsurgical drains, and extracorporeal membrane oxygenation.
Time-dependent antibiotics, which reach maximal efficacy when their concentration exceeds the MIC for a longer time, are mostly hydrophilic, so they result underdosed when Vd and drug clearance are increased. Such is the case of β-lactams, whose antimicrobial activity is optimized by more frequent administration rather than higher doses. Studies in ICUs have demonstrated that extended (3–4 h) or continuous (24 h) infusions of β-lactam antibiotics have equivalent or improved outcomes compared to intermittent (0.5–1 h) infusions, without increased adverse events.
5.3. Patients with Altered Renal Clearance
Dose adjustment is routinely recommended in patients with impaired renal function. Nevertheless, critically ill patients often present an augmented renal clearance (ARC) with a 130–160 mL/min creatinine clearance, due to aggressive fluid resuscitation, increased cardiac output, vasopressor use, and enhanced kidney blood flow. Patients with ARC are more likely to be younger (age < 50 years), male, have a modified Sequential Organ Failure Assessment Score (SOFA < 4 or less), and be admitted because of trauma [154]. This condition requires antimicrobial dose adjustments to avoid subtherapeutic concentrations, especially for β-lactams. Unfortunately, dose increase in these patients is not standard practice.
Another condition that may profoundly affect antibiotic dosing is continuous renal replacement therapy (CRRT), used for AKI management in hemodynamically unstable critically ill patients. Indeed, severe sepsis and septic shock are among the two most common reasons for CRRT initiation. CRRT can differ in modalities, hemofilters, and effluent rates, all of which may require dosing adjustments [156].
5.4. Obese Patients
Clinical studies report conflicting results about the impact of obesity on mortality in critically ill sepsis patients. Obesity is known as a chronic inflammation state that is related to increased oxidative stress [159]. A multitude of physiologic changes affecting PK and PD can occur in obese patients and may be responsible for antibiotic treatment failure due to lower serum concentration.
5.5. Burn Patients
Burn patients represent a particular population of critically ill patients. They are more susceptible to acquiring infections, and sepsis is the most important cause of mortality (rates of sepsis-related death are 50–84% in adult burn patients) [162]. This increased susceptibility has been attributed to some causes such as a nonspecific immunosuppressive state induced by burns (myeloid maturation arrest causing neutropenia, compromised cytotoxic T lymphocyte response, impaired neutrophil function, and decreased macrophage production [163]), loss of skin protection, respiratory injury from smoke, and frequent use of invasive devices (tracheal intubation, intravascular and urinary catheters) [164]. The leading cause of sepsis in these patients is the infection of burn wounds, and the most common isolated organisms are Pseudomonas aeruginosa and methicillin-resistant Staphylococcus aureus [162].
5.6. Adjunctive Therapies
Since sepsis and septic shock are characterized by a dysfunction of the immune response, with an initial increase in proinflammatory cytokines and a subsequent immune-paralysis, adjunctive immune-modulatory treatments have been developed in support of antibiotic therapies to restore immune response. Single adjunctive therapies have been studied and are currently being evaluated in clinical trials, with discordant results. Nevertheless, an observational study by Marik et al. showed a synergistic effect of a combination of intravenous vitamin C, thiamine, and hydrocortisone, resulting in a reduction in organ dysfunction and mortality of patients with septic shock [167].
6. Take-Home Messages
In Figure 2 are reported most important take home messages from this entry.
Figure 2. “Take home messages” about the main points treated in this review.
-
The mechanisms of sepsis are mainly based on the activation of a hyperinflammatory innate immune system response to infective stimuli and consequent endothelial activation and humoral changes, but it mostly relies on immunosuppression mechanisms involving both the innate and the adaptive immune systems.
-
The gold-standard diagnostic laboratory technique for the diagnosis of sepsis remains blood cultures.
-
Procalcitonin is an important tool to differentiate sepsis from noninfectious diseases and thereby contribute to early diagnosis.
-
Prompt empirical broad-spectrum antibiotic therapy and source control of infection are the most effective treatment strategy in sepsis.
-
Pharmacokinetic/pharmacodynamic adjustments are recommended for patients with specific characteristics (obesity, burns, altered renal function).
This entry is adapted from the peer-reviewed paper 10.3390/ijms23020803
This entry is offline, you can click here to edit this entry!
