Diabetic foot ulcer (DFU) is a devastating complication, affecting 15% of diabetic patients and representing the leading cause of non-traumatic amputations. Notably, the risk of mixed bacterial–fungal infection is elevated and highly associated with wound necrosis and poor clinical outcomes. Antimicrobial peptides (AMPs) are endogenous peptides that are naturally abundant in several organisms, such as bacteria, amphibians and mammals. These molecules have shown broad-spectrum antimicrobial activity and some of them even have wound-healing activity, establishing themselves as ideal candidates for treating multi-kingdom infected wounds.
- antimicrobial peptides
- chronic non-healing wounds
- diabetic foot ulcers
- wound healing
- bacterial and fungal infections
- biofilms
1. Introduction
2. Diabetic Foot Infection
3. Antimicrobial Peptides
|
AMP |
Primary Structure |
Length (aa) |
PDB Code |
Secondary Structure |
Tertiary Structure |
MW (Da) |
pI |
Net Charge |
Hydrophob. (kcal/mol) |
|
hBD-1 |
DHYNCVSSGG QCLYSACPIF TKIQGTCYRG KAKCCK |
36 |
1IJU |
α-helix + β-strand |
three antiparallel β-sheets stabilized by three disulfide bridges and flanked by an α-helix segment, together stabilized by a disulfide bridge |
3932 |
8.55 |
+4 |
+28.98 |
|
hBD-2 |
GIGDPVTCLK SGAICHPVFC PRRYKQIGTC GLPGTKCCKKP |
41 |
1FD4 |
4331 |
9.26 |
+6 |
+32.25 |
||
|
hBD-3 |
GIINTLQKYY CRVRGGRCAV LSCLPKEEQI GKCSTRGRKC CRRKK |
45 |
N.F. |
5158 |
10.47 |
+11 |
+45.26 |
||
|
LL-37 |
LLGDFFRKSK EKIGKEFKRI VQRIKDFLRN LVPRTES |
37 |
2K6O |
α-helix |
one α-helical conformation |
4491 |
11.15 |
+6 |
+41.03 |
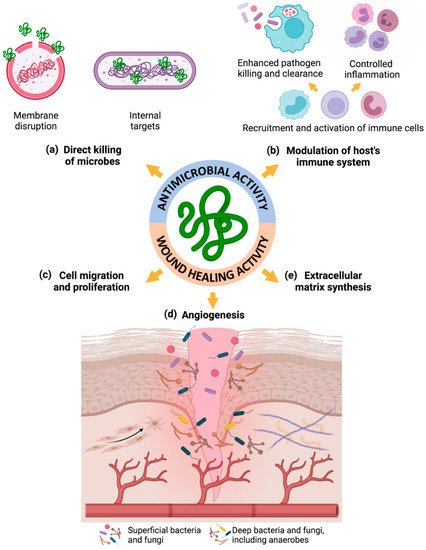
4. Changes of Endogenous AMPs in DFUs
5. Endogenous and Synthetic AMPs as Promising Therapeutic Agents for Infected Wounds
|
AMP |
Sequence |
Source |
Delivery Method |
Role in Antimicrobial and Wound-Healing Activities |
Ref. |
|
hBD-2
LL-37 |
GIGDPVTCLK SGAICHPVFC PRRYKQIGTC GLPGTKCCKKP LLGDFFRKSK EKIGKEFKRI VQRIKDFLRN LVPRTES |
Endogenous (human) Endogenous (human) |
Free
Free |
↑ antimicrobial activity (E. coli) ↑ keratinocyte migration |
[23] |
|
CW49 |
APFRMGICTTN |
Synthetic (frog skin) |
Free |
↑ angiogenic ability ↑ anti-inflammatory effect little effect on re-epithelialization |
[61] |
|
IDR-1018 |
VRLIVAVRIWRR-NH2 |
Synthetic |
Free |
↓ in vitro toxicity compared to LL-37 ↑ wound healing in S. aureus infected porcine and non-diabetic but not in diabetic murine wounds |
[62] |
|
IDR-1018 |
VRLIVAVRIWRR-NH2 |
Synthetic |
Free |
↑ angiogenic ability ↑ anti-inflammatory effect ↑ migration of endothelial cells |
[57] |
|
Pexiganan |
GIGKFLKKAK KFGKAFVKILKK |
Synthetic (analogue of magainin II—frog skin) |
Free |
↑ antimicrobial activity (E. coli, E. cloacae, Citrobacter spp., P. vulgaris, M. morganii, K. pneumoniae, S. marcescens, P. aeruginosa, A. baumannii, S. agalactiae, S. pyogenes, E. faecium, MSSA and MRSA) |
[58] |
|
3.1-PP4 |
KKLLKWLLKL LKTTKS |
Synthetic |
Free (chemically modified) |
↓ toxicity to HFF-1 human fibroblasts ↑ antimicrobial activity (E. coli, P. aeruginosa, and K. pneumoniae, including MDR isolates) ↓ formation of K. pneumoniae biofilms |
[66] |
|
PP4-3.11 |
KTTKSKKLLK WLLKLL |
Synthetic |
Free (chemically modified) |
↑ antimicrobial activity (Gram-positive and Gram-negative bacteria, including MDR isolates, as well as against relevant Candida spp.) |
[67] |
|
A-hBD-2 |
APKAMVTCLK SGAICHPVFC PRRYKQIGTC GLPGTKCCKKP |
Synthetic |
Free (chemically modified) |
↑ structural stability ↓ toxicity to keratinocytes ↑ antimicrobial activity (S. aureus) ↑ migration and proliferation of keratinocytes ↓ terminal differentiation of keratinocytes ↑ mobilization of intracellular Ca2+ ↑ wound healing in vivo |
[26] |
|
LFcinB |
FKCRRWQWRM KKLGAPSITC VRRAF |
Synthetic (derived from bLF) |
Free (chemically modified) |
↑ keratinocyte migration in vitro and ex vivo ↑ wound healing ↑ antimicrobial activity (B. pumilus and S. aureus) ↑ angiogenesis and collagen deposition ↓ inflammation |
[68] |
|
SHAP1 |
APKAMKLLKK LLKLQKKGI |
Synthetic |
Free (chemically modified) |
↓ toxicity to human erythrocytes and keratinocytes ↑ stability to proteases exposure ↑ wound closure compared to LL- 37 in vitro ↑ healing in vivo full-thickness excisional wounds ↑ antimicrobial activity (S. aureus) ↑ healing in S. aureus-infected murine wounds |
[69] |
|
SR-03791 |
MLKLIFLHRL KRMRKRLDLysRK |
Synthetic |
Free (chemically modified) |
↑ proliferation of human dermal fibroblasts ↑ antimicrobial activity (bacteria, including drug-resistant, and also fungi, namely: E. coli, P. aeruginosa, S. aureus, C. krusei, T. mentagrophytes, T. rubrum, MRSA and A. baumannii (MDR)) ↑ accelerated wound healing in two different wound-healing rat models |
[47] |
|
AMP |
Sequence |
Source |
Delivery Method |
Role in Antimicrobial and Wound-Healing Activities |
Ref. |
|
hBD-1
HNP-1 |
GNFLTGLGHR SDHYNCVSSG GQCLYSACPI FTKIQGTCYR GKAKCCK EPLQARADEV AAAPEQIAAD IPEVVVSLAW DESLAPKHPG SRKNMACYCR IPACIAGERR YGTCIYQGRLWAFCC |
Endogenous (human)
Endogenous (human) |
Niosomal gel
Niosomal gel |
↑ antimicrobial activity (MRSA-infected wound in rats and MSSA and MRSA isolated from patients with DFIs) |
[70] |
|
Nisin |
ITSISLCTPG CKTGALMGCN MKTATCH(or N)CSIHVSK |
Endogenous (bacteria) |
Guar gum gel |
↑ antimicrobial activity against S. aureus DFU biofilm-producing isolates, including some MDR clinical isolates |
[25] |
|
Nisin |
ITSISLCTPG CKTGALMGCN MKTATCH(or N)CSIHVSK |
Endogenous (bacteria) |
Guar gum gel |
↑ antibacterial activity against biofilms formed by DFI S. aureus |
[71] |
|
aCT1 2 |
RQPKIWFPNR RKPWKKRPRP DDLEI-acid |
Synthetic (analogue of Cx43) |
Hydroxyethyl cellulose gel |
↓ ulcer area in DFU patients ↑ ulcer re-epithelialization in DFU patients ↓ time-to-complete-ulcer closure in DFU patients |
[56] |
|
ASP-1 ASP-2 |
RRWVRRVRRW VRRVVRVVRRWVRR RWWRWWRRWWRR |
Synthetic |
Gel, Stratex or PU-based dressings |
↑ eradication of mono- and polymicrobial biofilms of MDR pathogens: S. aureus, A. baumannii, K. pneumoniae, P. aeruginosa, and MRSA ↑ BI compared to free ASP-1 and ASP-2 |
[72] |
|
IKYLSVN |
IKYLSVN |
Synthetic |
GOx-loaded hydrogel |
↑ antimicrobial activity (S. aureus) ↓ blood glucose concentration of diabetic patients |
[73] |
|
LL-37 |
LLGDFFRKSK EKIGKEFKRI VQRIKDFLRN LVPRTESC |
Synthetic |
Gold-nanoscale formulation |
↑ phosphorylation of EGFR and ERK1/2 ↑ migratory properties of keratinocytes ↑ wound-healing activity in vivo ↑ expression of collagen, IL6 and VEGF |
[48] |
|
Pexiganan 2 |
GIGKFLKKAK KFGKAFVKILKK |
Synthetic (analogue of magainin II—frog skin) |
Cream |
= clinical outcome, microbiological eradication (S. aureus, E. coli, E. cloacae, S. marcescens, P. aeruginosa, Enterococcus spp., MSSA and MRSA), and wound healing as ofloxacin ↓ bacterial resistance in vivo |
[74] |
|
Cys-KR12 |
CKRIVKRIKKWLR |
Synthetic (originated from LL37) |
SF nanofiber membrane (chemically modified) |
↑ antimicrobial activity (S. aureus, S. epidermidis, E. coli, and P. aeruginosa) ↑ proliferation of keratinocytes and fibroblasts ↑ differentiation of keratinocytes ↓ LPS-induced TNF-α expression of monocytes |
[75] |
|
K11R-K17R1 |
DSHAKRHHGY RRKFHERHHSHRGY |
Synthetic (analogue of Hst-5 peptide) |
HPMC-based bioadhesive hydrogel (chemically modified) |
↑ antimicrobial activity (C. albicans strains resistant to traditional antifungals) ↑ cell proliferation and migration in human oral keratinocytes |
[76] |
|
KSL-W |
KKVVFWVKFK |
Synthetic (analogue of KSL peptide) |
Pluronic F-127 gel (chemically modified) |
↑ antibiofilm and antimicrobial activity (chronic wound infection biofilm-embedded bacteria, including MRSA, S. epidermidis, CoNS, and A. baumannii) |
[77] |
|
TC19 |
LRCMCIKWWSG KHPK |
Synthetic (derived from human TC-1-derived peptide L3) |
HPMC gel (chemically modified) |
↓ toxicity to human fibroblasts ↑ antimicrobial activity (ESKAPE panel in vitro, and MRSA and A. baumannii (MDR) in a murine superficial wound infection model) ↓ bacterial resistance inflammation in vitro |
[78] |
|
Tet213 |
KRWWKWWRRC |
Synthetic (cysteinylated HHC36 peptide) |
Alg/HA/Col dressing (chemically modified) |
↑ antimicrobial activity (E. coli, S. aureus, MRSA) ↑ proliferation of NIH 3T3 fibroblast cells ↑ wound healing, re-epithelialization, collagen deposition, and angiogenesis in vivo rat model of partial-thickness mixed-bacterial infected wounds |
[79] |
6. Conclusion and Future Perspectives
Despite a wealth of research about AMPs and their respective application as potential therapy for non-healing infected wounds, this area needs further investigation. There is evidence that the performance of chemical modifications and the use of delivery systems can greatly improve the characteristics of AMPs to be applied as alternatives to antibiotics and antifungals. Indeed, these strategies can protect AMPs from host diabetic microenvironment, protease degradation and serum inactivation, reduce their inherent toxicity and improve their targeting and prolonged delivery. Accordingly, AMP-based approaches could be a solution for the emergence of antimicrobial resistance or could be applied in association with antibiotics or antifungals to promote a synergistic action for treating chronic wounds. However, few have been developed to treat polymicrobial infections that include anaerobic bacteria, fungi, and biofilms, and consequently to improve the treatment of infected DFUs. Only Gomes et al., Tomioka et al., and Sultan et al. have evaluated the action of PP4-3.1, SR-0379 and K11R-K17R against fungi, respectively, without any study considering the action of AMPs against anaerobic bacteria present in the DFU microenvironment. Therefore, further studies will need to include more models of infection with anaerobic bacteria, fungi, and biofilms, since infected DFUs tend to have a multi-kingdom basis. It is noteworthy that the infection models used in the different studies presented herein include microorganisms that are more pathogenic and predominant in DFUs, such as S. aureus (MSSA and MRSA), P. aeruginosa, E. coli, and A. baumannii, as well as some Candida spp. However, these infection models only include one or two of these microbes, and do not consider the complexity of polymicrobial infections and biofilms in human-infected chronic wounds. Furthermore, more accurate models of infected DFUs need to be included in future research to prove the efficacy of novel AMP delivery systems as therapeutic approaches for treating chronic infected wounds. Indeed, better wound models also need to be implemented to better mimic the human condition, including full-thickness infected wound models. Together, these future improvements could conduct to a greater translation into the clinical practice and consequently to a reduction of clinical trial failure rates, leading to effective management and treatment approaches for multi-kingdom infected DFUs, to enhance the health and the quality of life of these patients.
This entry is adapted from the peer-reviewed paper 10.3390/biom11121894
