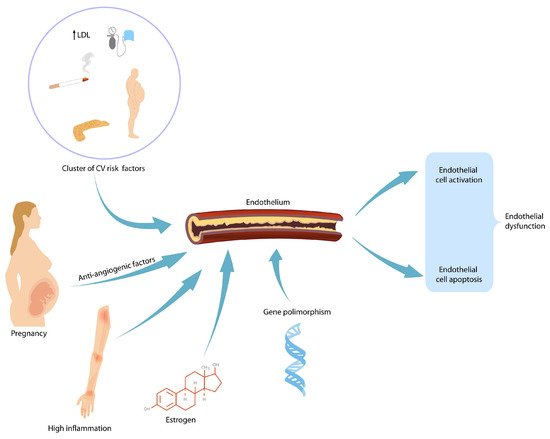
Preeclampsia (PE) is a pregnancy-related complication affecting multiple maternal organs characterized by new-onset hypertension after 20 weeks. In previously healthy pregnancies that are complicated by PE, pre-existing, subclinical cardiovascular risk factors are usually identified: maternal obesity [
10], smoking, elevated lipid levels [
11], hypertension [
12], insulin resistance [
13] and thrombophilia [
14]. In patients who develop PE before 32 weeks, trophoblastic invasion is suboptimal, suggesting decreased uteroplacental blood flow [
15]. High resistance in the uteroplacental flow is evident long before the clinical onset of high blood pressure. The poorly perfused placenta appears to be the source of anti-angiogenic factors that affect maternal vascular endothelium and fenestration of glomerular capillaries, causing proteinuria [
16]. Women with high blood pressures in early pregnancy appear to be more sensitive to the circulating anti-angiogenic factor soluble fms-like tyrosine kinase-1 (sFlt-1) and, therefore, are at higher risk of developing PE [
17]. First trimester screening for PE with accurate prophylactic measurements [
18] and efficient treatment when hypertensive disorders are detected [
19] can improve pregnancy outcomes and reduce the risk of long-term cardiovascular consequences. A vulnerability to future cardiovascular disease after PE has been described for many years [
20]. Systematic reviews have put together numerous studies on the long-term consequences of PE and quantified the risk of future cardiovascular disease (CVD) after pregnancy with and without PE [
21]. After one pregnancy is affected by PE, there is a 6 times increased chance of having a recurrent ischemic attack within a year after developing acute coronary syndrome [
21]. Melchiorre et al. describe a so-called “dose-dependent” effect of hypertensive disorders of pregnancy (HDPs) and the long-term risk of developing chronic hypertension depending on the severity of PE, the onset of complications during gestation, the necessity of iatrogenic preterm birth, the association with intrauterine fetal growth restriction (FGR) and the number of gestations affected by HDPs [
22]. After at least two pregnancies with PE, there is a 3 times increased risk of developing hypertension [
23] as well as a shorter lifespan [
24]; an increased chance of stroke (hazard ratio 5.10) [
25]; an increased chance of ischemic heart disease (hazard ratio 3.3) [
25]; and increased rates of heart failure, cerebrovascular accident and hospitalization because of cardiovascular disease [
26].
In patients with HDPs, heart failure is more likely to develop within 5 years postpartum [
27]. The associated risk of later CVD is lower for pregnancy-related hypertension than for PE but is still elevated (RR 1.9–2.5) [
28]. Thus, HDPs and PE in particular may be regarded as novel risk factors for women’s future cardiovascular health, with a constant need for long-term follow-up. There are data linking PE and diastolic heart failure, but whether the latter is a consequence of the former, or whether there is a common pathogenic background, is still under debate [
29,
30]. Research into early changes in diastolic function and follow-up on markers common to both pathologies (N-terminal pro-B natriuretic peptide—NT pro-BNP, high-density lipoprotein cholesterol [
30]) might be a worthwhile undertaking. The pathophysiological mechanism behind the increased subsequent risk of CVD is linked to endothelial dysfunction and structural abnormalities, including increased carotid intima–media thickness and accelerated coronary calcification and plaque deposition (
Figure 1) [
31]. This endothelial damage persists beyond the acute phase. However, whether this damage persists in the long term and is responsible for the increased number of cardiovascular events seen in this group is still up for debate. Preeclamptic pregnancies are characterized by increased CD 34+ cells as a reaction to endothelial dysfunction [
32]. Whether the number of endothelial progenitor cells (EPC) can be linked to the severity of preeclampsia remains to be demonstrated.
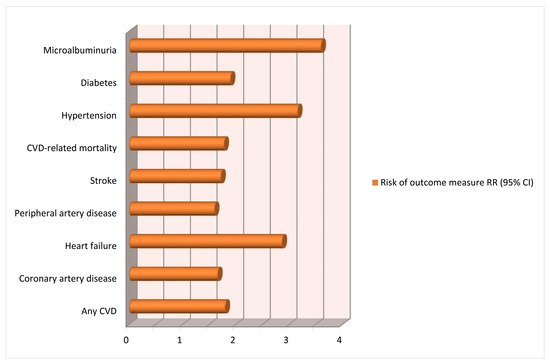
The latest meta-analysis included 73 studies (including cohort and case–control studies), analyzed over 13 million pregnancies and showed an important association between HDPs and long-term consequences on maternal cardiovascular system [
33]. Important heterogeneity was caused by all or part of the variables, including type of exposure, follow-up time, geographic region and sample source. This is most probably caused by the results from the various reviews performed over the years. We acknowledge the limitations of the meta-analysis in terms of heterogeneity of the included studies and lack of long-term follow-up (
Figure 2).