1. Analysis on Research Results
1.1. Sequence and Phylogenetic Analysis of SlPG49
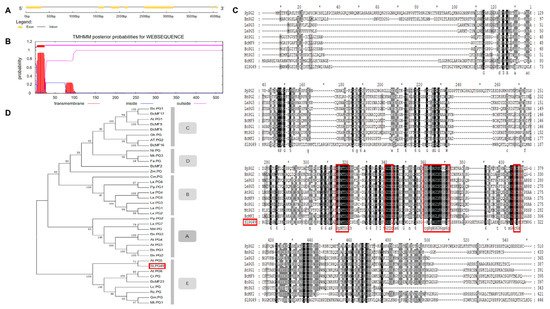
A PG gene (Solyc08g082170.2.1), named SlPG49, was isolated and cloned from Micro-Tom on the basis of the sequence available in Sol Genomics Network (solgenomics.net). SlPG49 contains seven exons and six introns (
Figure 1A), and encodes a protein of 452 amino acid residues (49.03 kD). Transmembrane domain analysis indicated that SlPG49 contains a transmembrane domain and belongs to the category of membrane localization proteins (
Figure 1B). To determine the evolutionary position of SlPG49, a phylogenetic tree containing SlPG49 and 37 PGs (
Table S2) from other species was constructed. The results showed that SlPG49 belongs to clade E, and clustered with the other eight PGs, including AtPG5 in Arabidopsis thaliana and BcMF23 in
Brassica rapa (
Figure 1C). Then, sequence alignment was performed on the nine PGs from clade E and the other four PGs from different clades. As expected, the nine PGs that belong to the clade E showed higher amino acid sequence similarity. Further analysis revealed that the PGs in clade E all contained conserved domains Ⅰ, Ⅱ, and Ⅳ, but domain Ⅲ was less conserved (
Figure 1D).
Figure 1. Gene structure, transmembrane region, multiple alignment, and phylogenetic analysis of SlPG49: (A) gene structure of SlPG49; (B) transmembrane region of SlPG49; (C) phylogenetic analysis of SlPG49; (D) multiple alignment analysis of SlPG49.
1.2. Expression Pattern Analysis of SlPG49
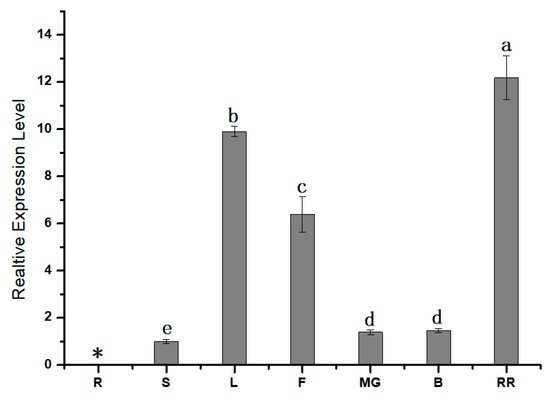
To further understand the role of SlPG49, qRT-PCR was first conducted to study the expression pattern of SlPG49 in tomato plants. The results showed that SlPG49 transcripts were detectable in all organs examined except roots. SlPG49 was highly expressed both in fruits of red ripening stage and leaves, but moderately accumulated in flowers, and weakly expressed in mature green fruits, breaker fruits, and stems (Figure 2). These findings suggested that SlPG49 may be crucial for tomato fruit softening, as well as leaf and flower development.
Figure 2. Expression pattern analysis of SlPG49 by qRT-PCR. R: roots; S: stems; L: leaves; F: flowers; MG: mature green fruit; B: breaker fruit; RR: red ripening fruit. Data are presented as mean ± SE with at least three biological replicates. Significant differences are indicated by different letters (p < 0.05). * indicates undetected expression level.
1.3. Subcellular Localization of SlPG49
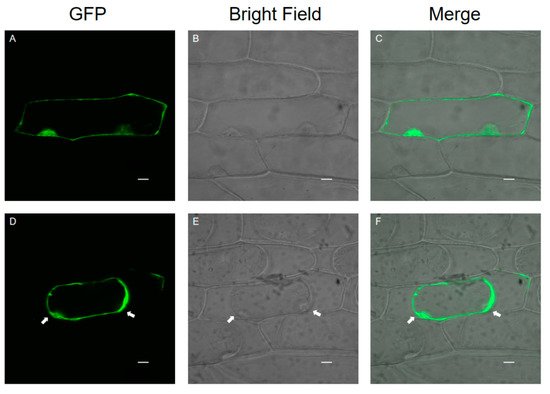
Onion epidermal cells are a good material for the transient expression of exogenous genes because of their clear structure, lack of chloroplasts, and convenient materials. A transient expression assay in onion epidermal cells with CaMV35S::SlPG49-GFP plasmids was conducted using particle bombardment to examine the subcellular localization of SlPG49. The results showed that green fluorescence signals were widely distributed in onion epidermal cells transformed with SlPG49-GFP (Figure 3A–C), and the fusion protein signals of SlPG49-GFP could be detected in the cell membrane, cell wall, and extracellular space in the plasma wall-isolated onion epidermal cells (Figure 3D,F). These results indicate that SlPG49 is a secreted protein, and localizes in the cell membrane and the cell wall.
Figure 3. Subcellular localization of SlPG49-GFP fusion protein in living onion epidermal cells: (A–C) localization of SlPG49-GFP; (D–F) localization of SlPG49-GFP in plasmolyzed onion epidermal cells; cells were plasmolyzed by treatment with 1 mol·L−1 mannitol for 5 min. White arrowheads show the membranes separated from the cell wall. Bars = 20 µm.
1.4. Enzymatic Activity of SlPG49 In Vitro
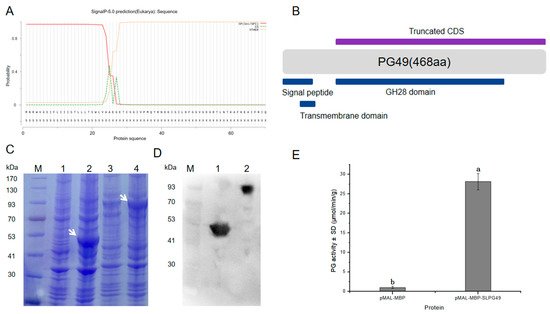
SlPG49 was heterologously expressed, and its activity was tested to characterize whether it encoded an active PG. SlPG49 was predicted to contain a signal peptide (Figure 4A). A truncated CDS, including sequences encoding GH28 domain, was cloned into the pMAL-c2x vector (Figure 4B) to improve protein solubility, using pMAL-MBP as a negative control. The predicted sizes of SlPG49 and MBP tag protein were 49.03 kDa and 43 kDa, respectively. The results of SDS-PAGE gels and Western blot illustrated that the sizes of the tag protein band and fusion protein band were consistent with the prediction (Figure 4C,D), indicating that SlPG49 protein was successfully isolated and purified. The purified proteins were incubated with polygalacturonic acid to analyze its hydrolytic activity. The results demonstrated that SlPG49 showed high enzymatic activity (Figure 4E), indicating that SlPG49 encodes a bona fide PG.
Figure 4. SlPG49 displays PG activity in vitro: (A,B) signal peptide analysis and schematic protein structure of SlPG49; (C) SDS-PAGE analysis; M, protein maker; 1, not induced pMAL-MBP; 2, induced pMAL-MBP; 3, not induced pMAL-MBP-SlPG49; 4, induced pMAL-MBP-SlPG49. White arrowheads show the bands of pMAL-MBP-SlPG49 and pMAL-MBP; (D) western blot analysis; 1: purified pMAL-MBP; 2: purified pMAL-MBP-SlPG49; (E) purified pMAL-MBP-SlPG49 shows PG activity in vitro. Data are presented as mean ± SE with at least three biological replicates. Significant differences are indicated by different letters (p < 0.05).
2. Current Insights
According to the latest classification system, PGs can be divided into seven clades (A–G), and most members of clade E can be detected at a high overall expression in all tissues [
45]. In the present work, phylogenetic analysis showed that
SlPG49 was located in clade E and closed to
ATPG5 (
Figure 1D). Interestingly, the expression of
ATPG5 was significantly increased when an
Arabidopsis endo-PG (
AtPGLR) was knocked out, indicating that
ATPG5 has the function of replenishing
AtPGLR, which may also be an endo-PG [
29]. Therefore, in view of the close evolutionary relationship between SlPG49 and ATPG5, we speculated that
SlPG49 was probably an endo-PG. As mentioned earlier, PG has four conserved domains (Ⅰ–Ⅳ). Generally, genes encoding the proteins of these four domains are defined as PG genes. Notably, domain Ⅲ is relatively poorly conserved [
46]. The results of multiple sequence alignments show that the conservation of domain III is indeed lower than that of domains Ⅰ, Ⅱ, and Ⅳ (
Figure 1D). Although they are different from the typical domain Ⅲ, the nine PGs from clade E (including SlPG49) are highly conserved in domain Ⅲ. It has been reported that the positively charged domain Ⅳ may participate in an ionic interaction with the carboxyl terminal of the substrate [
47]. Here, although R (Arginine) in the domain Ⅳ of SlPG49 is replaced by H (Histidine), it is still positively charged. Therefore, we speculated that the structure and function of SlPG49 may not have been changed.
A previous study showed that the enzymatic activity of PG increased during the ripening period of the tomato fruit, suggesting that these enzymes may be critical for initiating the ripening process [
21]. In our previous research,
SlPG49 was predicted to have a high expression in red ripening fruits [
18]. In this work, qPCR analyses confirmed that
SlPG49 is extremely highly expressed in fruits at the red ripening stage (
Figure 2), demonstrating that
SlPG49 may be involved in tomato fruit ripening and softening.
Pectin is synthesized in the Golgi apparatus and secreted into the cell wall in the state of methyl esterification and acetylation, and can be modified and degraded in the cell wall [
26]. PGs are synthesized in the endoplasmic reticulum and secreted into the cell wall after maturation in the trans-Golgi network [
48,
49]. In accordance with their function, it is predicted that PGs exhibit extracellular localization. Previous studies have demonstrated that a PG protein in
Brassica campestris, BcMF23, was a secreted protein and exhibited extracellular localization by subcellular localization of onion epidermis transient expression [
50]. In transgenic Arabidopsis,
ATPGX3 and
AtPG45 were located in the cell wall [
51,
52]. The present transient expression result in the onion epidermis confirmed that SlPG49 was a secreted protein and may play a great role in the cell wall or extracellular matrix (
Figure 3), similar to PGs in
A. thaliana. To date, only nine PG genes have been identified in
A. thaliana, with five that have been proven to show PG activity in vitro [
51,
53,
54,
55]. However, there is no report of in vitro activity of PGs in tomatoes. In this work, SlPG49 was successfully induced and purified by prokaryotic expression, and it was proved that SlPG49 has the activity of degrading polygalacturonic acid in vitro (
Figure 4E), demonstrating that SlPG49 is another bonafide PG. Studies have shown that the increase in PG activity promotes the degradation of pectin in the cell walls of strawberries, apples, bananas, and other fruits, and accelerates fruit ripening and softening [
37,
38,
39]. This study shows that the expression of SlPG49 increases significantly during the softening stage, and it has the activity of degrading one of the main components of pectin (polygalacturonic acid), indicating that it may play an important role in tomato fruit softening.
In summary, a new PG gene, SlPG49, has been identified in S. lycopersicum. The sequence analysis suggested that SlPG49 possesses the basic characteristics of PG family members. The results of expression pattern, subcellular localization, and enzymatic activity analysis indicated that SlPG49 may function in the degradation of cell wall polygalacturonic acid during fruit softening. Further studies will focus on elucidating the precise biological function and regulation of SlPG49 in fruit ripening.