Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biology
Taste processing is an adaptive mechanism involving complex physiological, motivational and cognitive processes. Animal models have provided relevant data about the neuroanatomical and neurobiological components of taste processing.
- flavour
- molecular signalling
- olfactory processing
- receptors
- taste learning
- taste processing
1. Introduction
Three important sensory systems depend on physical stimuli to initiate their processing. The somatosensory system recognises touch, pain and temperature; the auditory system recognises sound waves and the visual system recognises visual waves, as specific physical modalities. There are two other sensory systems which, however, depend on the stimulation produced by different chemical molecules. Olfactory and taste systems are activated by small particles stimulating specific receptor cells located in the respective membranes of the nose and tongue. Both mechanisms are critical for feeding and are therefore essential for the survival and evolution of species. Food releases odorant volatile particles that stimulate the olfactory system, allowing the recognition of food and the identification of any potential danger. Additionally, different chemical elements of food initiate the activation of the taste system in the lingual epithelium. This mechanism allows the identification of the five basic taste qualities associated with food, that is, sweet, salty, sour, bitter and umami [1][2][3].
2. Taste System
Taste buds of mammals’ oral cavity membranes contain dozens of clustered taste receptor cells selectively sensitive to one of the five basic taste qualities: sweet, salty, sour, bitter and umami [4]. Taste and visceral processing signals are sent by the VII, IX and X cranial nerves to different nuclei of the brainstem [5]. The nucleus of the solitary tract (NTS) is the first brainstem nucleus that receives taste afferents [5][6][7]. From this nucleus, taste information is ipsilaterally sent to another brainstem group of cells, the posteromedial parabrachial nucleus, and then ascending projections reach several brain structures, such as the lateral hypothalamus, the bed nucleus of the stria terminalis, the amygdala and the ventroposteromedial and lateral thalamus [8][9]. Finally, the higher level of taste processing takes place in the gustatory insular cortex [7][10][11]. This is the taste pathway described in rodents. However, in humans, the taste discrimination process might include mechanisms and systems parallel to taste detection [12]. Figure 1 depicts the main structures and pathways of taste processing in rodents. These systems, together with the pathways involved in visceral malaise processing via the vagus nerve (X cranial nerve), are necessary for taste learning, as well as taste aversion learning and memory [13]. Indeed, the involvement of different taste processing levels through the central nervous system makes possible the modulation of somatic and autonomic responses, as well as the control of the behavioural and hedonic responses of animals [14]. The relevance of taste learning for eating behaviours lies not only in the decision to search for some foods and avoid other foods due to the acquisition of learned taste preferences, but mainly because this decision can be vital, especially in omnivorous species, when food is potentially harmful, as is detailed later. For this reason, taste learning requires special attention.
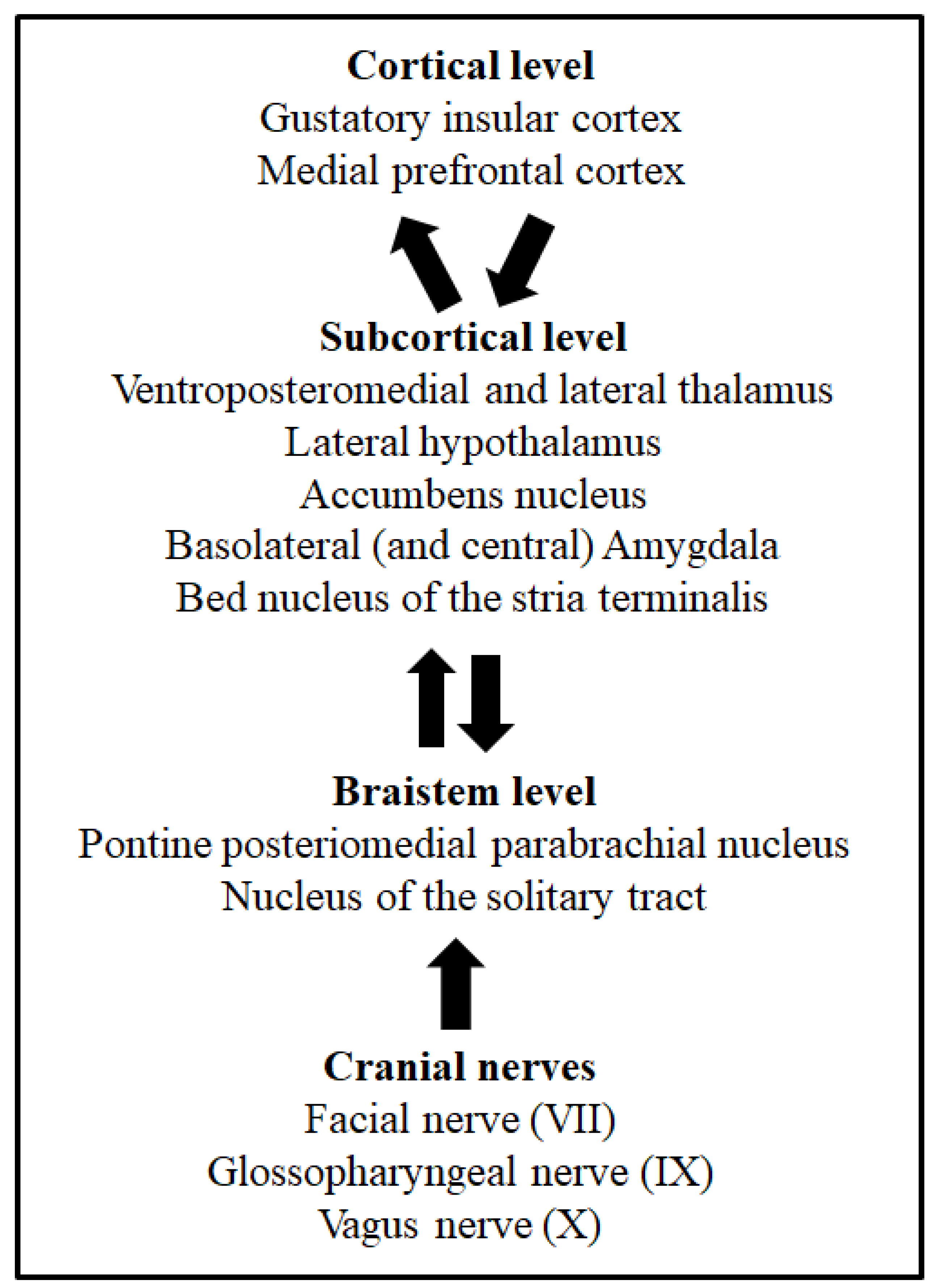
Figure 1. Cortical, subcortical, and brainstem levels of taste processing and taste learning. From bottom to top, arrows indicate the ascending taste pathway from the oral cavity and the afferent and efferent projections associated to taste learning. The cranial nerves involved in taste processing from the oral membranes are also shown.
Additionally, the olfactory system, which is responsible for odour processing, plays an essential role in taste processing and taste learning. Down-top odour processing involves olfactory sensory neurons [15], the olfactory nerve (I cranial nerve) [16], the olfactory bulbs and the olfactory tract [17]. The brain structures that process olfactory information are the piriform cortex, the olfactory tubercle, the periamygdaloid and lateral entorhinal cortices, the cortical region of the amygdaloid nuclei, the ventral tenia teat, the nucleus of the lateral olfactory tract, the anterior cingulate cortex, the insular cortex and the overlying operculum (including the somatomotor mouth region), and the orbitofrontal cortex [16][18]. The relevance of the olfactory system for taste processing and taste learning has been widely described in rodents [19][20].
3. Taste Learning
The ability to distinguish between different tastes is a learning related to survival. For example, the sweet taste of saccharin or glucose, which provides high calories and therefore energy [21][22], induces dopamine release in the reward system of the brain [23][24]. This system makes it possible to learn taste preferences as an adaptive mechanism in nature. Animal models have revealed numerous molecular processes and brain areas involved in taste learning [25].
3.1. Conditioned Taste Aversion: A Peculiar Taste Learning
Vertebrates and some invertebrates have developed neural systems that allow the association of non-familiar tastes with visceral toxic effects, resulting in a learned aversion (known as conditioned taste aversion or CTA) to tastes with similar taste characteristics [7]. The learned aversion results in an adaptative response of rejection to the toxic food that induced gastrointestinal malaise (thus reducing the probability of toxic experiences) and an acquired perception of unpleasant taste. This type of taste learning is useful for preserving life, particularly for omnivorous species, including human beings. The acquired taste aversion can be extinguished if further experiences with the same taste stimulus (or even similar taste stimuli) are not associated with poisoning or negative consequences.
CTA and CTA extinction can be reproduced in the laboratory with animal models in order to explore the neurobiological and behavioural characteristics of this learning [26][27]. In the laboratory (as in nature), CTA is characterised by the fact that taste aversion can be acquired through a single association between the taste stimulus and visceral malaise, and because the association between the novel taste and visceral consequences is possible even some hours after the processing of both stimuli [28][29]. This characteristic of CTA, to resist long delays between stimuli, is explained by the physiological processes of digestion, which implies a delay between the taste processing and visceral consequences. Considering this physiological time interval between the taste processing and the gastrointestinal malaise, CTA could result from the association between the taste memory’s trace and the visceral disease. The CTA paradigm used in the laboratory is a useful tool to investigate the biological and behavioural bases of aversive learning and taste memory, not only regarding the specificity of CTA but also with respect to other taste learning phenomena, such as neophobia (a precautionary response to the first exposure to a novel food or taste) or latent inhibition of CTA (a reduced conditioned aversion resulting from taste pre-exposures without negative or aversive consequences) [30][31][32][33]. An example of the relevance of the CTA paradigm is the finding that physical (external) and internal (such as the time of day) cues can separately modulate the magnitude of a learned aversion [34][35][36].
The conventional CTA paradigm includes ad libitum feeding throughout the procedure and a daily water restriction baseline stage to facilitate similar physiological states among the animals. The next stage is the conditioning session. In this stage, the animals are exposed to a novel taste stimulus (the conditioning stimulus, CS, in the terminology of associative learning). In the CTA paradigm, taste stimuli are usually provided as fluids (for example, as saccharin or sodium chloride solutions) to facilitate intake measurement. Around 20 min after intake, an intraperitoneal injection of lithium chloride (LiCl 0.15 M, 2% of body weight) is administrated to induce gastrointestinal malaise (the unconditioned stimulus, US). To a lesser extent, other aversive stimuli have also been used to induce taste aversion [37][38]. A recovery day with ad libitum water usually follows this stage. The magnitude of the taste aversion is evaluated the next day (testing day), by one-bottle (CS) or two-bottle (CS vs. water) tests, according to the amounts consumed. Figure 2 shows the CTA paradigm stages. This paradigm is used to explore the neurobiology of taste aversion learning and memory.
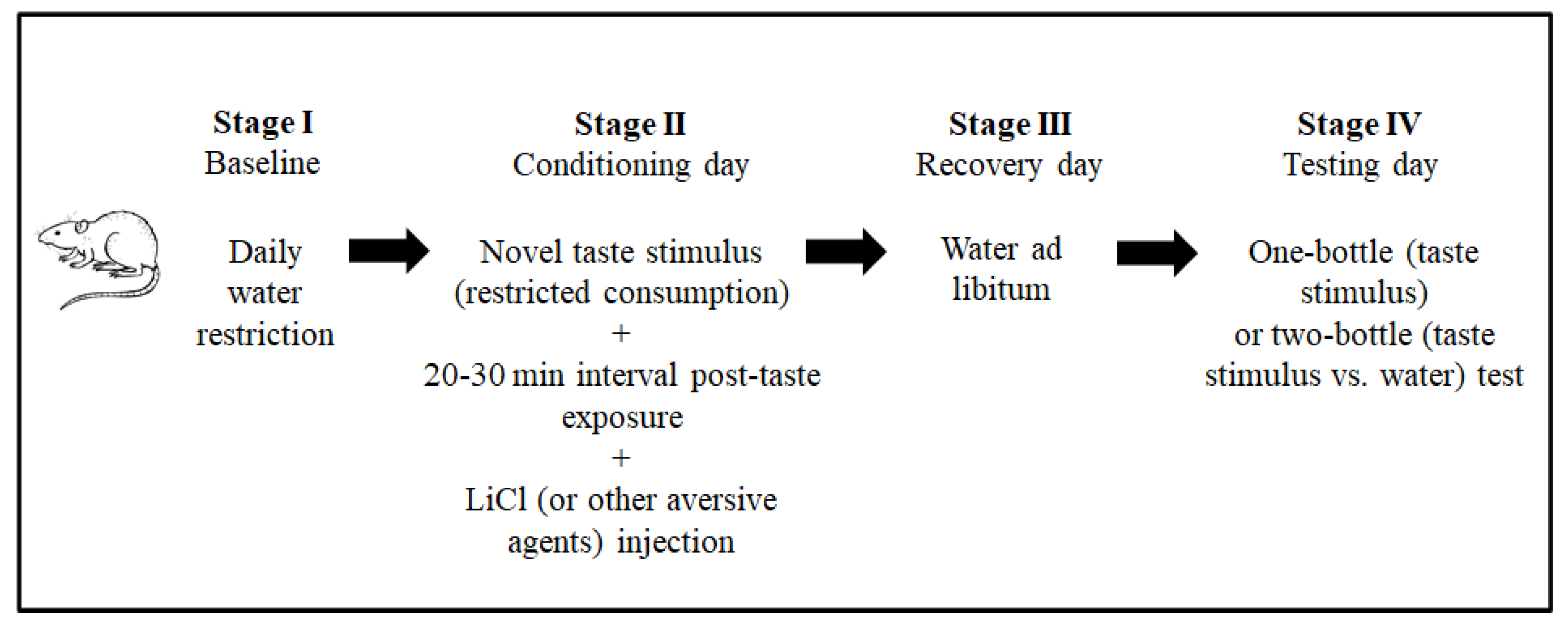
Figure 2. Principal stages of the procedure to induce conditioned taste aversion (CTA) in laboratory animal models.
3.2. Neural Network of CTA
A complex nervous system mechanism is involved in the acquisition of CTA [39][40]. Animal models provide critical data to understand the neuroanatomy and neurobiology of taste learning and memory. The neural network of taste aversion learning and memory includes the nucleus of the solitary tract (NTS), the posteromedial pontine parabrachial nucleus, the lateral hypothalamus, the bed nucleus of the stria terminalis, the amygdala and the ventroposteromedial and lateral thalamus. The superior cortical level of processing has been described in the gustatory insular cortex region. The functional connectivity between the parabrachial nucleus and the gustatory insular cortex is selectively involved in the acquisition of CTA but not in the formation of safe taste memories [41]. These pathways and the vagal system involved in the processing of visceral malaise are necessary for the acquisition of CTA and taste aversion memory [7][8][9][11]. Moreover, the role of other brain structures in the neurobiology of CTA, such as the medial prefrontal cortex and the nucleus accumbens, is being elucidated at present [42], together with the functions of the piriform [43] and perirhinal [44] cortices in taste recognition. The neural system of CTA involves the activity of this brain and brainstem network, but the specific functions of each component are not fully understood. Although the CTA mechanisms of the gustatory insular cortex and parabrachial nucleus are well described, the involvement of the amygdala and its nuclei in specific processes of taste aversion learning and memory is not fully known [45][46][47]. Animal lesion studies have pointed to the central and basolateral nuclei of the amygdala as the amygdaloid nuclei with specific functions in taste aversion learning and memory [48]. However, the basolateral amygdala seems to be the main nucleus involved in the acquisition of CTA [47], probably modulating the magnitude of taste aversion [49][50]. A possible mechanism by which basolateral amygdala can modulate the intensity of CTA is through the neophobia phenomenon, considering that this nucleus is implicated in the perception of novelty of taste stimuli. The correct processing of novelty is one of the mechanisms affecting the magnitude of CTA [51]. In addition to lesion studies, other methods and approaches have also pointed to the basolateral amygdala as a selective amygdaloid nucleus mediating the acquisition of CTA. By two-photon calcium imaging it has been revealed that a CTA-dependent neuronal activation of specific neurons of the insular cortex that project to the basolateral amygdala [52], and chemical activation of the insular cortex-basolateral amygdala projection by Clozapine-N-oxide after taste exposure, can induce aversive taste memory in mice [53]. Thus, the function of the basolateral amygdala on CTA might be controlled by afferent axons from the gustatory insular cortex [51]. Moreover, molecular studies have supported the relevance of this cortico-amygdaloid projection for the formation of CTA [54][55]. It can be concluded that specific connections between the gustatory insular cortex and the basolateral complex of the amygdala [47][55][56], and between the amygdala and the brainstem nuclei involved in CTA [9][45][46], could be recruited to influence the intensity of acquired taste aversions.
3.3. Molecular Mechanisms of CTA
Some molecular mechanisms are specific to certain forms of taste learning and memory [57]. However, learning of novel tastes, taste familiarity and taste aversion extinction share biological pathways and mechanisms with CTA. The transcriptional processes necessary for the acquisition of taste learning or processing of taste novelty that occur in the gustatory insular cortex have been described in rats [58]. Particularly, this model has provided relevant information about how novel taste experience, a process that strengthens the acquisition of CTA, modifies the genetic transcription in this cortical area during taste memory consolidation [58]. Likewise, taste learning of novel or familiar tastes promotes different changes in the transcriptome of this cortical region. Moreover, the consolidation of positive or negative taste learning (according to its positive or negative visceral consequences) also induces transcriptional activity in this region [58]. Learning of novel tastes induces biochemical alterations in the gustatory insular cortex of other rodents as well, including increased cholinergic activity [59], and changes in protein phosphorylation [60], facilitating taste memory consolidation [61][62]. Furthermore, several studies have shown that taste memory consolidation is altered after pharmacological inhibition of protein synthesis in the gustatory insular cortex [63][64]. Recently [65], the administration of protein synthesis inhibitors directly into the gustatory insular cortex during long-term taste memory acquisition altered the formation of long-term memory. However, this procedure did not affect memory persistence when these inhibitors were infused 3 days after the memory formation. Interestingly, the infusion of protein synthesis inhibitors 14 days after the memory acquisition increased the memory persistence [65], which suggests that long-term memory may be altered by protein synthesis inhibitors, even several days after the formation of taste memory.
Other molecular mechanisms of taste learning that could participate in CTA have been seen in the gustatory insular cortex. Various immediate early genes identified in this cortical region during taste learning, such as the activity-regulated cytoskeleton associated protein (Arc)/Arg3.1 gene, seem to regulate the excitability of synapses associated with synaptic plasticity processes and long-term taste memory [66][67]. The function of the Arc/Arg3.1 protein appears to be different depending on the specificity of taste learning, since novel taste learning can increase as well as reduce the expression of this protein in the gustatory insular cortex, according to specific time points. These transcriptional changes may last for hours and are more intense compared to the processing of familiar tastes [58]. Moreover, a hemispheric lateralisation of the expression of Arc/Arg3.1 protein is observed in the gustatory insular cortex related to the processing of novel tastes [68].
The neural plasticity mechanisms related to taste learning, including CTA, also involve other molecular sequences. The expression of brain-derived neurotrophic factor (BDNF) in the gustatory insular cortex (and the basolateral amygdala) induces long-term synaptic plasticity, and the acquisition of taste aversions seems to block the long-lasting BDNF-induced strengthening of synaptic plasticity [69]. These findings point to the BDNF gene expression in the gustatory insular cortex as one of the molecular mechanisms critically involved in the long-term synaptic plasticity processes related to taste memory. Additional gene expressions have been found in the gustatory insular cortex during taste learning, including the expression of c-fos [70], Homer1a [71] and the transcription factor Elk-1 [72]. The functions of these gene expressions and their respective proteins are unclear, although it is assumed that they are a relevant part of the synaptic plasticity necessary for taste learning and memory [73]. All this evidence indicates that the gene expression in the gustatory insular cortex and in other brain regions involved in taste learning is a molecular key for the acquisition of taste learning and the subsequent taste memory [61].
This entry is adapted from the peer-reviewed paper 10.3390/molecules25143112
References
- Roper, S.D.; Chaudhari, N. Taste buds: Cells, signals and synapses. Nat. Rev. Neurosci. 2017, 18, 485–497.
- Palmer, R.K. A pharmacological perspective on the study of taste. Pharmacol. Rev. 2019, 71, 20–48.
- Ohla, K.; Yoshida, R.; Roper, S.D.; Di Lorenzo, P.M.; Victor, J.D.; Boughter, J.D.; Fletcher, M.; Katz, D.B.; Chaudhari, N. Recognizing taste: Coding patterns along the neural axis in mammals. Chem. Senses 2019, 44, 237–247.
- Lee, H.; MacPherson, L.J.; Parada, C.A.; Zuker, C.S.; Ryba, N.J.P. Rewiring the taste system. Nature 2017, 548, 330–333.
- Harrer, M.I.; Travers, S.P. Topographic organization of Fos-like immunoreactivity in the rostral nucleus of the solitary tract evoked by gustatory stimulation with sucrose and quinine. Brain Res. 1996, 711, 125–137.
- Spray, K.J.; Bernstein, I.L. Afferent and efferent connections of the parvicellular subdivision of iNTS: Defining a circuit involved in taste aversion learning. Behav. Brain Res. 2004, 154, 85–97.
- Scott, T.R. Learning through the taste system. Front. Syst. Neurosci. 2011, 5, 87.
- Dayawansa, S.; Ruch, S.; Norgren, R. Parabrachial-hypothalamic interactions are required for normal conditioned taste aversions. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2014, 306, 190–200.
- Bielavska, E.; Roldan, G. Ipsilateral connections between the gustatory cortex, amygdala and parabrachial nucleus are necessary for acquisition and retrieval of conditioned taste aversion in rats. Behav. Brain Res. 1996, 81, 25–31.
- Small, D.M. Taste representation in the human insula. Brain Struct. Funct. 2010, 214, 551–561.
- Stehberg, J.; Moraga-Amaro, R.; Simon, F. The role of the insular cortex in taste function. Neurobiol. Learn. Mem. 2011, 96, 130–135.
- Wallroth, R.; Ohla, K. As soon as you taste it: Evidence for sequential and parallel processing of gustatory information. eNeuro 2018, 5.
- Molero-Chamizo, A.; Nathzidy Rivera-Urbina, G. Molecular mechanisms involved in taste learning and memory. AIMS Mol. Sci. 2017, 4, 389–397.
- Lunceford, B.E.; Kubanek, J. Reception of aversive taste. Integr Comp Biol. 2015, 55, 507–517.
- Reed, R.R. Signaling pathways in odorant detection. Neuron 1992, 8, 205–209.
- Wackermannová, M.; Pinc, L.; Jebavý, L. Olfactory sensitivity in mammalian species. Physiol. Res. 2016, 65, 369–390.
- Tham, W.W.P.; Stevenson, R.J.; Miller, L.A. The functional role of the medio dorsal thalamic nucleus in olfaction. Brain Res. Rev. 2009, 62, 109–126.
- Small, D.M.; Green, B.G. A proposed model of a flavor modality. In The Neural Bases of Multisensory Processes; Murray, M.M., Wallace, M.T., Eds.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2012; ISBN 9781439812198.
- Sakai, N.; Imada, S. Bilateral lesions of the insular cortex or of the prefrontal cortex block the association between taste and odor in the rat. Neurobiol. Learn. Mem. 2003, 80, 24–31.
- Fanselow, M.S.; Birk, J. Flavor-flavor associations induce hedonic shifts in taste preference. Anim. Learn. Behav. 1982, 10, 223–228.
- De Araujo, I.E.; Ferreira, J.G.; Tellez, L.A.; Ren, X.; Yeckel, C.W. The gut-brain dopamine axis: A regulatory system for caloric intake. Physiol. Behav. 2012, 106, 394–399.
- Sclafani, A.; Ackroff, K. Flavor preferences conditioned by nutritive and non-nutritive sweeteners in mice. Physiol. Behav. 2017, 173, 188–199.
- Han, W.; Tellez, L.A.; Perkins, M.H.; Perez, I.O.; Qu, T.; Ferreira, J.; Ferreira, T.L.; Quinn, D.; Liu, Z.W.; Gao, X.B.; et al. A neural circuit for gut-induced reward. Cell 2018, 175, 887–888.
- Lenoir, M.; Serre, F.; Cantin, L.; Ahmed, S.H. Intense sweetness surpasses cocaine reward. PLoS ONE 2007, 2, e698.
- Ferreira, G.; Ferry, B.; Meurisse, M.; Lévy, F. Forebrain structures specifically activated by conditioned taste aversion. Behav. Neurosci. 2006, 120, 952–962.
- Molero-Chamizo, A. Taste aversions. In Encyclopedia of Animal Cognition and Behavior; Springer: Berlin, Germany, 2018; pp. 1–7.
- Bernstein, I.L. Taste aversion learning: A contemporary perspective. Nutrition 1999, 15, 229–234.
- Bures, J.; Bermudez-Rattoni, F.; Yamamoto, T. Ethology, physiological psychology, and neurobiology of CTA. In Conditioned Taste Aversion: Memory of a Special Kind; Oxford University Press: New York, NY, USA, 1998; pp. 1–10.
- Bures, J.; Bermudez-Rattoni, F.; Yamamoto, T. The CTA paradigm: Terminology, methods, and conventions. In Conditioned Taste Aversion: Memory of A Special Kind; Bures, J., Bermúdez-Rattoni, F., Yamamoto, T., Eds.; Oxford University Press: New York, NY, USA, 1998; pp. 14–25.
- Molero-Chamizo, A.; Moron, I. Latent inhibition of conditioned taste aversion in rats with excitotoxic dorsal hippocampal lesions. J. Neurosci. Res. 2015, 93, 1740–1747.
- Roman, C.; Lin, J.Y.; Reilly, S. Conditioned taste aversion and latent inhibition following extensive taste preexposure in rats with insular cortex lesions. Brain Res. 2009, 1259, 68–73.
- Molero-Chamizo, A. Excitotoxic lesion of the hippocampus of Wistar rats disrupts the circadian control of the latent inhibition of taste aversion learning. Brain Res. 2013, 1533, 105–113.
- Molero-Chamizo, A. Excitotoxic lesion of the posterior part of the dorsal striatum does not affect the typically dopaminergic phenomenon of latent inhibition in conditioned taste aversion. Neurosci. Res. 2015, 91, 8–12.
- Molero-Chamizo, A.; Rivera-Urbina, G.N. Effects of temporal contexts and contextual habituation on latent inhibition. Psicothema 2017, 29, 346–351.
- Molero-Chamizo, A. Circadian-temporal context and latent inhibition of conditioned taste aversion: Effect of restriction in the intake of the conditioned taste stimulus. Learn. Behav. 2016, 45, 157–163.
- Molero-Chamizo, A. Changes in the time of day of conditioning with respect to the pre-exposure interfere with the latent inhibition of conditioned taste aversion in rats. Behav. Processes 2018, 146, 22–26.
- Traverso, L.M.; Ruiz, G.; De La Casa, L.G. MK-801 induces a low intensity conditioned taste aversion. Pharmacol. Biochem. Behav. 2012, 100, 645–651.
- St. John, S.J.; Pour, L.; Boughter, J.D. Phenylthiocarbamide produces conditioned taste aversions in mice. Chem. Senses 2005, 30, 377–382.
- Yamamoto, T.; Ueji, K. Brain mechanisms of flavor learning. Front. Syst. Neurosci. 2011, 5, 76.
- Yamamoto, T. Neural mechanisms of taste aversion learning. Neurosci. Res. 1993, 16, 181–185.
- Clark, E.W.; Bernstein, I.L. Establishing aversive, but not safe, taste memories requires lateralized pontine-cortical connections. Behav. Brain Res. 2009, 197, 356–363.
- Marotta, R.; Fenu, S.; Scheggi, S.; Vinci, S.; Rosas, M.; Falqui, A.; Gambarana, C.; De Graziella, M.G.; Acquas, E. Acquisition and expression of conditioned taste aversion differentially affects extracellular signal regulated Kinase and glutamate receptor phosphorylation in rat prefrontal cortex and nucleus accumbens. Front. Behav. Neurosci. 2014, 8, 153.
- Grau-Perales, A.; Gómez-Chacón, B.; Morillas, E.; Gallo, M. Flavor recognition memory related activity of the posterior piriform cortex in adult and aged rats. Behav. Brain Res. 2019, 360, 196–201.
- Morillas, E.; Gómez-Chacón, B.; Gallo, M. Flavor and object recognition memory impairment induced by excitotoxic lesions of the perirhinal cortex. Neurobiol. Learn. Mem. 2017, 144, 230–234.
- Reilly, S.; Bornovalova, M.A. Conditioned taste aversion and amygdala lesions in the rat: A critical review. Neurosci. Biobehav. Rev. 2005, 29, 1067–1088.
- Schafe, G.E.; Bernstein, I.L. Forebrain contribution to the induction of a brainstem correlate of conditioned taste aversion: I. The amygdala. Brain Res. 1996, 741, 109–116.
- St. Andre, J.; Reilly, S. Effects of central and basolateral amygdala lesions on conditioned taste aversion and latent inhibition. Behav. Neurosci. 2007, 121, 90–99.
- Yamamoto, T.; Sako, N.; Sakai, N.; Iwafune, A. Gustatory and visceral inputs to the amygdala of the rat: Conditioned taste aversion and induction of c-fos-like immunoreactivity. Neurosci. Lett. 1997, 226, 127–130.
- Molero-Chamizo, A.; Rivera-Urbina, G.N. Effects of lesions in different nuclei of the amygdala on conditioned taste aversion. Exp. Brain Res. 2017, 235, 3517–3526.
- Molero-Chamizo, A. Modulation of the magnitude of conditioned taste aversion in rats with excitotoxic lesions of the basolateral amygdala. Neurobiol. Learn. Mem. 2017, 137, 56–64.
- Reilly, S. Central gustatory system lesions and conditioned taste aversion. In Conditioned Taste Aversion: Behavioral and Neural Processes; Reilly, S., Schachtman, T.R., Eds.; Oxford University Press: New York, NY, USA, 2009; pp. 309–327.
- Lavi, K.; Jacobson, G.A.; Rosenblum, K.; Lüthi, A. Encoding of conditioned taste aversion in cortico-amygdala circuits. Cell Rep. 2018, 24, 278–283.
- Kayyal, H.; Yiannakas, A.; Kolatt Chandran, S.; Khamaisy, M.; Sharma, V.; Rosenblum, K. Activity of insula to basolateral amygdala projecting neurons is necessary and sufficient for taste valence representation. J. Neurosci. 2019, 39, 9369–9382.
- Bermúdez-Rattoni, F.; Ramírez-Lugo, L.; Gutiérrez, R.; Miranda, M.I. Molecular signals into the insular cortex and amygdala during aversive gustatory memory formation. Cell. Mol. Neurobiol. 2004, 24, 25–36.
- Miranda, M.I.; McGaugh, J.L. Enhancement of inhibitory avoidance and conditioned taste aversion memory with insular cortex infusions of 8-Br-cAMP: Involvement of the basolateral amygdala. Learn. Mem. 2004, 11, 312–317.
- Ming, T.K.; Bernstein, I.L. Mapping conditioned taste aversion associations using c-Fos reveals a dynamic role for insular cortex. Behav. Neurosci. 2005, 119, 388–398.
- Vincis, R.; Fontanini, A. Central taste anatomy and physiology. Handb Clin Neurol. 2019, 164, 187–204.
- Inberg, S.; Jacob, E.; Elkobi, A.; Edry, E.; Rappaport, A.; Simpson, T.I.; Armstrong, J.D.; Shomron, N.; Pasmanik-Chor, M.; Rosenblum, K. Fluid consumption and taste novelty determines transcription temporal dynamics in the gustatory cortex. Mol. Brain 2016, 9, 13.
- Miranda, M.I.; Ferreira, G.; Ramírez-Lugo, L.; Bermúdez-Rattoni, F. Role of cholinergic system on the construction of memories: Taste memory encoding. Neurobiol Learn Mem. 2003, 80, 211–222.
- Stern, E.; Chinnakkaruppan, A.; David, O.; Sonenberg, N.; Rosenblum, K. Blocking the eIF2α kinase (PKR) enhances positive and negative forms of cortex-dependent taste memory. J. Neurosci. 2013, 33, 2517–2525.
- Gal-Ben-Ari., S.; Rosenblum, K. Molecular mechanisms underlying memory consolidation of taste information in the cortex. Front. Behav. Neurosci. 2012, 5, 87.
- Elkobi, A.; Ehrlich, I.; Belelovsky, K.; Barki-Harrington, L.; Rosenblum, K. ERK-dependent PSD-95 induction in the gustatory cortex is necessary for taste learning, but not retrieval. Nat. Neurosci. 2008, 11, 1149–1151.
- Rosenblum, K.; Meiri, N.; Dudai, Y. Taste memory: The role of protein synthesis in gustatory cortex. Behav. Neural Biol. 1993, 59, 49–56.
- Merhav, M.; Rosenblum, K. Facilitation of taste memory acquisition by experiencing previous novel taste is protein-synthesis dependent. Learn. Mem. 2008, 15, 501–507.
- Levitan, D.; Gal-Ben-Ari, S.; Heise, C.; Rosenberg, T.; Elkobi, A.; Inberg, S.; Sala, C.; Rosenblum, K. The differential role of cortical protein synthesis in taste memory formation and persistence. NPJ Sci. Learn. 2016, 1, 16001.
- Okuno, H. Regulation and function of immediate-early genes in the brain: Beyond neuronal activity markers. Neurosci. Res. 2011, 69, 175–186.
- Plath, N.; Ohana, O.; Dammermann, B.; Errington, M.L.; Schmitz, D.; Gross, C.; Mao, X.; Engelsberg, A.; Mahlke, C.; Welzl, H.; et al. Arc/Arg3.1 is essential for the consolidation of synaptic plasticity and memories. Neuron 2006, 52, 437–444.
- Inberg, S.; Elkobi, A.; Edri, E.; Rosenblum, K. Taste familiarity is inversely correlated with Arc/Arg3.1 hemispheric lateralization. J. Neurosci. 2013, 33, 11734–11743.
- Rivera-Olvera, A.; Rodríguez-Durán, L.F.; Escobar, M.L. Conditioned taste aversion prevents the long-lasting BDNF-induced enhancement of synaptic transmission in the insular cortex: A metaplastic effect. Neurobiol. Learn. Mem. 2016, 130, 71–76.
- Doron, G.; Rosenblum, K. C-Fos expression is elevated in GABAergic interneurons of the gustatory cortex following novel taste learning. Neurobiol. Learn. Mem. 2010, 94, 21–29.
- Saddoris, M.P.; Holland, P.C.; Gallagher, M. Associatively learned representations of taste outcomes activate taste-encoding neural ensembles in gustatory cortex. J. Neurosci. 2009, 29, 15386–15396.
- Berman, D.E. Modulation of taste-induced Elk-1 activation by identified neurotransmitter systems in the insular cortex of the behaving rat. Neurobiol. Learn. Mem. 2003, 79, 122–126.
- Bermúdez-Rattoni, F. Molecular mechanisms of taste-recognition memory. Nat. Rev. Neurosci. 2004, 5, 209–217.
This entry is offline, you can click here to edit this entry!
