Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Hematology
T-cell lymphomas are a relatively rare group of malignancies with a diverse range of pathologic features and clinical behaviors. Recent molecular studies have revealed a wide array of different mechanisms that drive the development of these malignancies and may be associated with resistance to therapies.
- PTCL
- non-Hodgkin’s lymphoma
- novel therapies
- targeted therapies
- recent advances
- current treatment
1. Introduction
Peripheral T-cell lymphomas (PTCLs) are a diverse set of aggressive T-cell lymphomas that arise from mature T cells [1]. Most PTCLs are associated with a poor prognosis [2]. PTCLs constitute 15–20% of aggressive non-Hodgkin’s lymphomas (NHLs) and 5–10% of all NHLs [3]. PTCL has a diverse morphology, and definitive markers of PTCL subtypes are scarce, making the diagnosis and classification of PTCL complex [4]. The 2016 World Health Organization classification system describes 27 different types of PTCL [5].
Most PTCLs are treated similarly due to the lack of specific targeted therapies for different PTCL subtypes. This, in turn, is due to an inadequate understanding of the pathobiology of these tumors. However, recent advances in whole-genome sequencing and gene expression profiling (GEP) have enabled us to better elucidate the pathogenesis of PTCL and differentiate among its various subtypes [6]. Recent studies have uncovered individual molecular signatures that may not only provide prognostic information about the disease but may also provide potential therapeutic targets. Multiple promising therapies are being tested and based on evidence from clinical trials; these therapies are being incorporated into the currently recommended treatment regimens.
2. Novel Targeted Therapies
This section highlights the novel targeted agents being studied alone or in combination with other drugs for the treatment of PTCL. Some of these drugs, along with their molecular targets, are included in Figure 1. The results from the clinical trials evaluating these agents are summarized in Table 1.
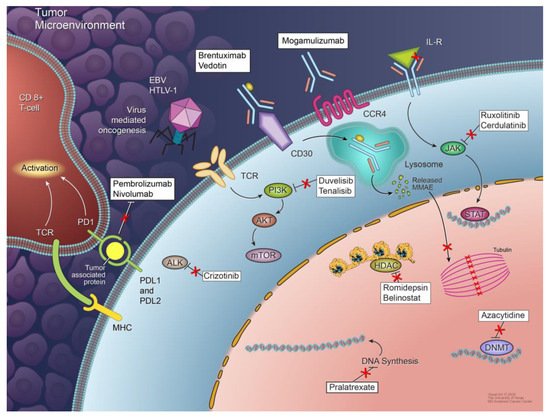
Figure 1. Mechanisms of peripheral T-cell lymphoma development and agents used for targeted therapies. Multiple mechanisms are responsible for the development and proliferation of different subtypes of PTCL, including signaling pathway deregulation, epigenetic dysregulation, tumor microenvironment signals, and virus-mediated oncogenesis. Not all of these mechanisms are involved in the pathogenesis of each PTCL subtype. Some potential therapeutic targets and agents targeting them are also shown.
Table 1. Results of clinical trials of targeted agents for the treatment of peripheral T-cell lymphoma.
| Target | Therapy | Trial Phase | N | ORR | CRR | Survival | Grade ≥ 3 AEs | Ref |
|---|---|---|---|---|---|---|---|---|
| CD30 | BV + CHP | III | 226 | 83% | 68% | 3-year PFS: 57.1% | Neutropenia, anemia, diarrhea, peripheral neuropathy | [7] |
| PI3Kγ/δ | Duvelisib | I | 16 | 50% | 18.8% | mPFS: 8.3 months mOS: 8.4 months |
Transaminase increases, neutropenia, maculopapular rash | [8] |
| PI3Kγ/δ and HDAC | Duvelisib + Romidepsin | I | 27 | 59% | 33% | NR | Neutropenia, elevated transaminases, hyponatremia | [9] |
| PI3Kγ/δ and Proteasome | Duvelisib + Bortezomib | I | 16 | 44% | 25% | NR | Neutropenia, elevated transaminases, hyponatremia | [9] |
| PI3Kγ/δ | Tenalisib | I/Ib | 35 (PTCL and CTCL) | 45.7% | 8.6% | NR | Fatigue, elevated transaminases, diarrhea | [10] |
| Proteasome | Bortezomib | II | 15 (ATLL) | 6.7% | 0% | PFS: 38 days | Thrombocytopenia, lymphopenia, leukopenia, peripheral neuropathy | [11] |
| JAK1 and JAK2 | Ruxolitinib | II | 27 (PTCL and CTCL) | 30% | 4% | NR | Neutropenia, lymphopenia, anemia, thrombocytopenia | [12] |
| SYK, JAK1, and JAK3 | Cerdulatinib | IIa | 26 | 35% | 31% | NR | Lipase increases, amylase increases, neutropenia | [13] |
| mTOR | Everolimus | II | 16 (PTCL and CTCL) | 44% | NR | mPFS: 8.5 months mOS: 10.2 months |
Anemia, leukopenia, neutropenia, thrombocytopenia, hyperglycemia, hypertriglyceridemia | [14] |
| DNA methylation | Azacytidine | Retrospective | 12 | 75% | 50% | mPFS: 15 months mOS: 21 months |
Diarrhea | [15] |
| DNA methylation and HDAC | Azacytidine + Romidepsin | I | NR | 73% | 55% | NR | Thrombocytopenia, neutropenia | [16] |
| HDAC | Chidamide | II | 83 | 28% | 14% | mPFS: 2.1 months mOS: 21.4 months |
Thrombocytopenia, leukopenia, neutropenia | [17] |
| AAK | Alisertib | III | 138 | 33% | 18% | mPFS: 3.8 months mOS: 13.7 months |
Neutropenia, anemia, thrombocytopenia, stomatitis, diarrhea | [18] |
| ALK | Crizotinib | Retrospective | 26 (ALK-positive ALCL) | 83–90% | 80–83% | NR | Neutropenia | [9] |
| CCR4 | Mogamulizumab | II | 26 (ATLL) | 50% | 31% | mPFS: 5.2 months mOS: 13.7 months |
Lymphopenia, leukopenia, neutropenia, thrombocytopenia | [12] |
| CD25 | Camidanlumab tesirine | I | 19 (T-cell lymphoma) | 42.1% | 5.3% | NR | Exfoliative dermatitis, neuropathy, thyroiditis | [19] |
| CD52 | Alemtuzumab + CHOP | III | 123 | NR | 56% | 3-year PFS: 33% 3-year OS: 46% |
Leukopenia, anemia, thrombocytopenia | [13] |
| Immune modulation | Lenalidomide | I/II | 54 | 22% | 11% | mPFS: 2.5 months | Neutropenia, thrombocytopenia | [14] |
| PD-1 | Pembrolizumab | Retrospective | 7 (NKTCL) | 100% | 71% | NR | - | [20] |
| PD-1 | Pembrolizumab | II | 13 | 33% | 27% | mPFS: 3.2 months mOS: 10.6 months |
Rash, pneumonitis | [21] |
| Immune modulation | Etoposide + CHOP | Retrospective | 609 | - | NA | mPFS: 4.9 months 5-year survival: 17.9% |
Anemia, thrombocytopenia | [22] |
| Immune modulation | Etoposide + CHOP | Retrospective | 20 | - | 50% | mPFS: 9 months 5-year survival: 5% |
Anemia, thrombocytopenia, neutropenia | [22] |
2.1. T-Cell and Costimulatory Receptor Signaling Inhibitors
The TCR and costimulatory receptor pathways, which are often altered in PTCL, are potential therapeutic targets. In one study, eight out of 12 AITL patients had a response to cyclosporine A, a calcineurin inhibitor that blocks TCR signaling, and three of these patients had a complete remission [23]. VAV1 activation accelerates TCR signaling, and dasatinib, a multikinase inhibitor, has been shown to block VAV1 activation in vitro and improves OS in mouse models. Dasatinib targets the Src kinase and, thus, could be therapeutically targeted in PTCLs bearing FYN mutations. Mutations in CD28, a costimulatory receptor, are correlated with a poor prognosis, and CD28-CTLA4 fusion has been identified in PTCL. Thus, ipilimumab, an anti-CTLA4 monoclonal antibody, could be studied as targeted therapy [24].
2.2. PI3K Inhibitors
The PI3Kγ and PI3Kδ isoforms are often required for the TCR-mediated activation of T cells. Duvelisib, an inhibitor of both PI3K isoforms, elicited an ORR of 50% and CRR of 18.8% in a phase I trial enrolling 16 patients with R/R PTCL. The most common grade 3 or 4 adverse events (AEs) were transaminase increases, neutropenia, and maculopapular rash [25]. Duvelisib, in combination with either romidepsin or bortezomib for the treatment of R/R PTCL, is currently being studied in a phase Ib/II trial. A preliminary analysis revealed that, in 27 patients, duvelisib plus romidepsin elicited an ORR of 59% and CRR of 33%, with tolerable side effects. Interestingly, a subset of eight AITL patients had an ORR of 75% and CRR of 63%. Frequent grade 3 or 4 AEs were neutropenia, elevated transaminase levels, and hyponatremia [8]. Another dual PI3Kγ/δ inhibitor, tenalisib, was found to be tolerable and had encouraging efficacy in a phase I/Ib study enrolling patients with R/R PTCL or CTCL. For 35 evaluable patients, the ORR was 45.7%. Among the PTCL patients, the ORR was 46.7%, and the CRR was 20%. Common AEs were fatigue, elevated transaminase levels, and diarrhea [9].
2.3. Proteasome Inhibitors
The proteasome inhibitor bortezomib, which inhibits NF-κB, has shown promise in the treatment of ATLL. In a phase II trial of bortezomib in 15 patients with R/R ATLL, one patient had a partial remission, and five patients had a stable disease [10]. All patients experienced one or more AEs, the most common of which were fever and thrombocytopenia. Grade 3 or higher AEs included thrombocytopenia, lymphopenia, leukopenia, and peripheral neuropathy [10]. Other trials are investigating the use of bortezomib combined with CHEP (cyclophosphamide, doxorubicin, etoposide, and prednisone) for untreated PTCL and the use of a similar agent, ixazomib, combined with romidepsin for R/R PTCL [24].
2.4. JAK-STAT Pathway and SYK Inhibitors
The JAK-STAT pathway is a potential therapeutic target in PTCL. The JAK inhibitor ruxolitinib in the treatment of PTCL and CTCL is under investigation. In one study that enrolled 33 patients with PTCL or CTCL, of whom 27 were evaluable, ruxolitinib elicited a 30% ORR and 4% CRR. Grade 3 or more drug-related AEs included neutropenia, lymphopenia, anemia, and thrombocytopenia [11]. SYK, a receptor tyrosine kinase, is another promising target, as it is expressed in 94% of PTCLs [12]. In preclinical studies, the SYK inhibitor R406 efficiently initiated apoptosis and repressed proliferation in T-cell lymphomas [26]. Cerdulatinib, a dual SYK/JAK inhibitor, has also exhibited substantial efficacy and tolerability in a phase IIa clinical trial in patients with R/R PTCL, eliciting an ORR of 35% and CRR of 31%. Frequently observed AEs were diarrhea, nausea, neutropenia, and elevated lipase and amylase levels [19].
2.5. mTOR Inhibitor
An inhibitor of the mTOR pathway, everolimus, has significant activity against the proliferation of malignant T cells in vitro. In a phase II study of everolimus in 16 patients with R/R PTCL or CTCL of different subtypes, the ORR was 44%, and the median PFS and OS durations were 8.5 months and 10.2 months, respectively. Among the patients whose disease responded to everolimus, the median response duration was 8.5 months. Grade 3 or 4 hematologic toxicities that were possibly related to everolimus included anemia, leukopenia, neutropenia, and thrombocytopenia. Nonhematologic grade 3 or more toxicities reported with this agent included hyperglycemia, hypertriglyceridemia, and hypercholesterolemia [13].
2.6. Epigenetic Modulators
DNA methylation genes are mutated in multiple PTCL subtypes and are frequently mutated in AITL. Therefore, hypomethylating drugs and HDAC inhibitors could prove to be effective against these diseases. In one study, a group of patients with R/R AITL had sustained responses to the hypomethylating agent azacytidine, with an ORR of 75% and CRR of 50%. The drug was well-tolerated, and only one patient developed grade 3 diarrhea [14]. Moreover, investigators have reported that combinations of hypomethylating drugs and HDAC inhibitors have a marked activity against PTCL. In a phase I multicenter study in PTCL patients, the combination of azacytidine and romidepsin was well-tolerated, eliciting an ORR of 73% and CRR of 55%. The dose-limiting toxicities seen were grade 3 or more thrombocytopenia and neutropenia [15]. Azacytidine is currently being investigated in combination with CHOP, pralatrexate, durvalumab, and bortezomib in clinical trials [27].
Chidamide, another HDAC inhibitor, for the treatment of R/R PTCL was investigated in a phase II study in China. The study enrolled 83 patients with AITL, ALCL, PTCL-NOS, or NKTCL. The ORR was 28%, and the CRR was 14%. Although the median PFS duration was only 2.1 months, the median OS duration was 21.4 months. AITL patients had the highest ORR (50%) and CRR (40%). Most AEs were grade 1 or 2 events. Grade 3 or more AEs were thrombocytopenia, leucopenia, and neutropenia [16].
2.7. Aurora A Kinase Inhibitor
Aurora A kinase, which is overexpressed in aggressive lymphomas, plays a vital role in mitosis during cell cycle progression. In the phase III LUMIERE trial, the Aurora A kinase inhibitor alisertib was compared with a choice of romidepsin, pralatrexate, or gemcitabine. Patients receiving alisertib had an ORR of 33%, a CRR of 18%, and median PFS and OS durations of 3.8 months and 13.7 months, respectively. An improved response with alisertib was, however, not demonstrated. Frequent grade 3 or more AEs related to alisertib included neutropenia, anemia, thrombocytopenia, stomatitis, and diarrhea [17]. However, the combination of romidepsin and alisertib was investigated in a phase I study that included four patients with R/R PTCL but elicited no response [18].
2.8. ALK Inhibitor
The ALK1 inhibitor crizotinib was investigated in 26 pediatric patients with R/R ALK-positive ALCL. In the two dosage groups evaluated, the ORRs were 83% and 90%, respectively, and the CRRs were 80% and 83%, respectively. Neutropenia was the most frequently reported grade 3 or more AE [28]. ALK-positive ALCL, which is resistant to crizotinib, could be treated with a blockade of platelet-derived growth factor receptor-β (PDGFRB), which has been shown to encourage lymphoma development and tumor propagation in mouse models of ALCL with NPM-ALK fusions. Imatinib, which inhibits both PDGFRB and PDGFRA, was reported to induce a complete remission in a patient with refractory ALK-positive ALCL [29]. Recently, the FDA approved crizotinib in children and young adults with relapsed/refractory ALCL after the results from a trial with 26 patients [30].
2.9. Anti-CCR4 Monoclonal Antibody
An anti-CCR4 monoclonal antibody, mogamulizumab, has elicited effective antitumor responses in PTCL cell lines and mouse models of ATLL [31]. In Japan, the agent is approved for the treatment of ATLL based on the results of a multicenter phase II study, which revealed the agent’s encouraging efficacy and tolerable side effects in patients with relapsed aggressive CCR4-positive ATLL. For the 28 patients enrolled in the study, the ORR was 50%, the CRR was 31%, and the PFS and OS durations were 5.2 months and 13.7 months, respectively. The most frequent AEs were skin rashes and infusion reactions, which were manageable. Most grade 3 or 4 AEs were hematologic and included lymphopenia, leukopenia, neutropenia, and thrombocytopenia [32]. The FDA has approved mogamulizumab for the treatment of R/R mycosis fungoides and Sezary syndrome but not for the treatment of PTCL-NOS [27].
2.10. Anti-CD25 Antibody
Camidanlumab tesirine (ADCT-301) is a conjugate of an anti-CD25 (also called IL-2 receptor subunit α) antibody and a pyrrolobenzodiazepine dimer toxin (tesirine) whose internalization through the IL-2 receptor results in cell death [33]. A multicenter phase I trial of camidanlumab tesirine enrolled 44 patients with R/R NHL, including 22 with T-cell lymphoma. For the 19 evaluable T-cell lymphoma patients, the ORR was 42.1%, and the CRR was 5.3% [34].
2.11. Anti-CD52 Antibody
Multiple PTCL subtypes express CD52, a glycoprotein present on the surfaces of T- and B-lymphocytes and NK cells. Although such expression warrants the investigation of alemtuzumab, an anti-CD52 antibody, for the treatment of PTCL, the expression of CD52 on normal T cells and B cells has limited the potential therapeutic use of alemtuzumab and raises concerns of a possible immunosuppression. Nevertheless, researchers conducted a pooled analysis of two phase III trials of CHOP plus alemtuzumab versus CHOP alone in 252 untreated PTCL patients. The CRR of the CHOP-plus alemtuzumab group (56%) was higher than that of the CHOP-alone group (43%), but the combination was not found to confer a benefit in the PFS and OS. Grade 3 or 4 hematologic toxicities were more common in the alemtuzumab group and included leukopenia, anemia, and thrombocytopenia [18][35].
2.12. Immunomodulator
An immunomodulatory drug, lenalidomide, has activity in the therapy of B-cell NHL. In the phase I/II EXPECT trial of lenalidomide of 54 patients with R/R PTCL, the. patients were treated with a median of three prior therapies. For all patients, the ORR was 22%, and the CRR was 11%. AITL patients had a higher ORR (31%) and CRR (15%). The PFS duration was 2.5 months overall but 4.6 months specifically among AITL patients. The common grade 3 or 4 hematologic toxicities were neutropenia and thrombocytopenia. Thirty-five percent of the patients experienced at least one AE that led to a dose reduction or interruption. Life-threatening AEs were reported in 54% of the patients; 12 patients died during the study [36].
2.13. Bispecific Antibodies
Molecularly engineered antibodies fall into two broad categories: immunotoxins and bispecific antibodies. The former has two arms; one that recognizes a tumor cell marker, and the other is bound to a toxin or drug. The latter are designed to recognize two molecular targets [37]. An anti-CD30/CD16 bispecific antibody, AFM13, directs NK cells against CD30-positive tumors. However, its efficacy against T-cell lymphomas has not been established [27]. Bispecific antibodies have also been designed to link a component of the TCR complex to a tumor surface marker, thereby directing T-cell cytotoxic activity towards the tumor [38]. A CD19/CD3 bispecific antibody, blinatumomab, yielded durable responses in patients with B-cell NHL, spurring interest in this emerging modality [39].
2.14. Checkpoint Inhibitors
PD-L1 and PD-1 are constitutively expressed in multiple PTCL subtypes and on host cells in the tumor microenvironment. The inhibition of these immune checkpoint molecules has shown a therapeutic efficacy against NHL [40]. In one retrospective study of seven patients with R/R NKTCL, pembrolizumab, an anti-PD-1 antibody, displayed high efficacy against the disease, with an ORR of 100% (7/7) and CRR of 71% (5/7). After a median of seven therapy cycles and a median follow-up of 6 months, all five patients who had complete remission still had no evidence of the disease [41]. In a phase II study in patients with R/R PTCL, pembrolizumab elicited modest activity, with an ORR of 33%, a CRR of 27%, and PFS and OS durations of 3.2 months and 10.6 months, respectively. The most common AE was a rash, and the most common grade 3 or higher AEs were a rash and pneumonitis [20]. A phase I/II study of a combination of pembrolizumab and romidepsin in 15 patients with R/R PTCL yielded early encouraging results, including an ORR of 44% and complete remission in three patients. Nausea, vomiting, and fatigue were the common grade 3 or more treatment-related AEs. Of note, two patients experienced hyper-progression of their disease within 10 days of treatment [21]. However, because PD-1 inhibits TCR signaling, there is some concern that its use in PTCL patients could result in lymphoma progression [42]. A report of rapid disease progression in ATLL patients receiving nivolumab supports this idea [43]. In one study, a patient developed PTCL secondary to treatment with a checkpoint inhibitor, possibly because PD-1 could also act as a tumor suppressor in T-cell lymphomas [44].
2.15. Inhibitors of Apoptosis
The inhibitor of apoptosis proteins (IAPs), which include cellular IAP (cIAP) and X-linked IAP (xIAP), have antiapoptotic functions and regulate cellular survival signaling pathways. XIAP inhibits caspases, which are involved in programmed cell death, and cIAP prevents the formation of proapoptotic signaling complexes [45]. The deregulation of IAP expression due to genetic alterations is seen in solid tumors and hematologic malignancies [46][47]. ASTX660, a novel non-peptidomimetic dual antagonist of cIAP1/2 and xIAP, has demonstrated clinical activity in a phase 1 clinical trial [48]. In a phase 2 trial, 16 peripheral T-cell lymphomas (PTCL) and 13 CTCL patients received ASTX at 180 mg/day on days 1–7 and 15–22 in a 28-day cycle. An ORR of 28% (by Lugano criteria) was seen in the 14 evaluable patients in the PTCL cohort, and three responding patients remained in the study. The most common AEs of any grade were subclinical lipase and amylase elevation. These results demonstrate the clinical activity of ASTX660 in T-cell lymphomas, and expansion of the phase 2 cohort is ongoing [49].
2.16. Chimeric Antigen Receptor T-Cell Therapy
Some researchers have encouraged the use of chimeric antigen receptor-T (CAR-T) cells as therapy for PTCL patients. Potential targets of such therapy include CD30, CD7, CD5, and CD4 [27]. Given the BV successful targeting of CD30, CD30 CAR-T cells seem particularly promising, and their effectiveness has been demonstrated in mouse models [50]. In a phase I trial in 18 patients with R/R Hodgkin’s lymphoma, CD30 CAR-T cells were shown to be safe, eliciting partial remission in seven patients and a stable disease in six patients [51]. In another phase I study of CD30 CAR-T cells in patients with CD30-positive disease, one of two patients with ALCL had a complete remission that lasted 9 months [52]. CAR-T cells targeting the TCR also seem promising. The effectiveness of CAR-T cells targeting the TCRβ chain has been documented [53]. CAR-NK cells have been investigated in preclinical studies, but clinical trials are required to validate their efficacy [54]. Developing CAR-T cells to target T-cell antigens seems promising; however, fratricide within a CAR-T-cell product might impede such a development. This issue might be addressed by using CRISPR technology to eliminate CAR-T cell targets in effector cells themselves [42].
This entry is adapted from the peer-reviewed paper 10.3390/cancers13225627
References
- Armitage, J.O. The aggressive peripheral T-cell lymphomas: 2017. Am. J. Hematol. 2017, 92, 706–715.
- Foss, F.M.; Zinzani, P.L.; Vose, J.M.; Gascoyne, R.D.; Rosen, S.T.; Tobinai, K. Peripheral T-cell lymphoma. Blood 2011, 117, 6756–6767.
- Patel, M. Peripheral T cell lymphoma. Indian J. Med. Res. 2018, 147, 439–441.
- Iqbal, J.; Wright, G.; Wang, C.; Rosenwald, A.; Gascoyne, R.D.; Weisenburger, D.D.; Greiner, T.C.; Smith, L.; Guo, S.; Wilcox, R.A.; et al. Gene expression signatures delineate biological and prognostic subgroups in peripheral T-cell lymphoma. Blood 2014, 123, 2915–2923.
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390.
- Iqbal, J.; Liu, Z.; Deffenbacher, K.; Chan, W.C. Gene expression profiling in lymphoma diagnosis and management. Best Pract. Res. Clin. Haematol. 2009, 22, 191–210.
- Sun, Y.; Li, L.; Li, X.; Zhang, L.; Wang, X.; Fu, X.; Sun, Z.; Zhang, X.; Li, Z.; Wu, J.; et al. Outcomes of GDPT (gemcitabine, cisplatin, prednisone, thalidomide) versus CHOP in newly diagnosed peripheral T-cell lymphoma patients. Ther. Adv. Med. Oncol. 2020, 12, 1758835920923829.
- Horwitz, S.M.; Moskowitz, A.J.; Jacobsen, E.D.; Mehta-Shah, N.; Khodadoust, M.M.S.; Fisher, D.C.; Myskowski, P.; Wang, E.B.; Tawa, R.M.; Davey, M.T.; et al. The Combination of Duvelisib, a PI3K-δ,γ Inhibitor, and Romidepsin Is Highly Active in Relapsed/Refractory Peripheral T-Cell Lymphoma with Low Rates of Transaminitis: Results of Parallel Multicenter, Phase 1 Combination Studies with Expansion Cohorts. Blood 2018, 132, 683.
- Huen, A.; Haverkos, B.M.; Zain, J.; Radhakrishnan, R.; Lechowicz, M.J.; Devata, S.; Korman, N.J.; Pinter-Brown, L.; Oki, Y.; Barde, P.J.; et al. Phase I/Ib Study of Tenalisib (RP6530), a Dual PI3K δ/γ Inhibitor in Patients with Relapsed/Refractory T-Cell Lymphoma. Cancers 2020, 12, 2293.
- Ishitsuka, K.; Utsunomiya, A.; Katsuya, H.; Takeuchi, S.; Takatsuka, Y.; Hidaka, M.; Sakai, T.; Yoshimitsu, M.; Ishida, T.; Tamura, K. A phase II study of bortezomib in patients with relapsed or refractory aggressive adult T-cell leukemia/lymphoma. Cancer Sci. 2015, 106, 1219–1223.
- Moskowitz, A.J.; Jacobsen, E.; Ruan, J.; Schatz, J.H.; Obadi, O.; Motylinski, K.; Jarjies, M.C.; Galasso, N.; Hancock, N.H.; Davey, M.T.; et al. Durable Responses Observed with JAK Inhibition in T-Cell Lymphomas. Blood 2018, 132, 2922.
- Feldman, A.L.; Sun, D.X.; E Law, M.; Novak, A.J.; Attygalle, A.D.; Thorland, E.C.; Fink, S.R.; A Vrana, J.; Caron, B.L.; Morice, W.G.; et al. Overexpression of Syk tyrosine kinase in peripheral T-cell lymphomas. Leukemia 2008, 22, 1139–1143.
- Witzig, T.E.; Reeder, C.; Han, J.J.; LaPlant, B.; Stenson, M.; Tun, H.W.; Macon, W.; Ansell, S.M.; Habermann, T.M.; Inwards, D.J.; et al. The mTORC1 inhibitor everolimus has antitumor activity in vitro and produces tumor responses in patients with relapsed T-cell lymphoma. Blood 2015, 126, 328–335.
- Lemonnier, F.; Dupuis, J.; Sujobert, P.; Tournillhac, O.; Cheminant, M.; Sarkozy, C.; Pelletier, L.; Marçais, A.; Robe, C.; Fataccioli, V.; et al. Treatment with 5-azacytidine induces a sustained response in patients with angioimmunoblastic T-cell lymphoma. Blood 2018, 132, 2305–2309.
- O’Connor, O.A.; Falchi, L.; Lue, J.K.; Marchi, E.; Kinahan, C.; Sawas, A.; Deng, C.; Montanari, F.; Amengual, J.E.; Kim, H.A.; et al. Oral 5-azacytidine and romidepsin exhibit marked activity in patients with PTCL: A multicenter phase 1 study. Blood 2019, 134, 1395–1405.
- Shi, Y.; Dong, M.; Hong, X.; Zhang, W.; Feng, J.; Zhu, J.; Yu, L.; Ke, X.; Huang, H.; Shen, Z.; et al. Results from a multicenter, open-label, pivotal phase II study of chidamide in relapsed or refractory peripheral T-cell lymphoma. Ann. Oncol. 2015, 26, 1766–1771.
- O’Connor, O.A.; Özcan, M.; Jacobsen, E.D.; Roncero, J.M.; Trotman, J.; Demeter, J.; Masszi, T.; Pereira, J.; Ramchandren, R.; Beaven, A.; et al. Randomized Phase III Study of Alisertib or Investigator’s Choice (Selected Single Agent) in Patients With Relapsed or Refractory Peripheral T-Cell Lymphoma. J. Clin. Oncol. 2019, 37, 613–623.
- Mulvey, E.; Ruan, J. Biomarker-driven management strategies for peripheral T cell lymphoma. J. Hematol. Oncol. 2020, 13, 1–20.
- Horwitz, S.M.; Feldman, T.A.; Hess, B.T.; Khodadoust, M.M.S.; Kim, Y.H.; Munoz, J.; Patel, M.R.; Phillips, T.J.; Smith, S.D.; Smith, S.M.; et al. The Novel SYK/JAK Inhibitor Cerdulatinib Demonstrates Good Tolerability and Clinical Response in a Phase 2a Study in Relapsed/Refractory Peripheral T-Cell Lymphoma and Cutaneous T-Cell Lymphoma. Blood 2018, 132, 1001.
- Barta, S.K.; Zain, J.; Macfarlane, A.W.; Smith, S.M.; Ruan, J.; Fung, H.C.; Tan, C.R.; Yang, Y.; Alpaugh, R.K.; Dulaimi, E.; et al. Phase II Study of the PD-1 Inhibitor Pembrolizumab for the Treatment of Relapsed or Refractory Mature T-cell Lymphoma. Clin. Lymphoma Myeloma Leuk. 2019, 19, 356–364.e3.
- Iyer, S.P.; Neelapu, S.S.; Burns, E.; Nair, M.R.; Hosing, C.; Nieto, Y.; Westin, J.R.; Parmar, M.S.; Fowler, N.H.; Nastoupil, M.L.J.; et al. A Phase I/II Study to Examine the Safety and Efficacy of Pembrolizumab 200 Mg Fixed Dose Administered Every 3 Weeks (Q3W) in Combination with Romidepsin in Relapsed or Refractory Peripheral T-Cell Lymphoma (PTCL). Blood 2019, 134, 1546.
- Kim, Y.A.; Byun, J.M.; Park, K.; Ae, K.Y.; Lee, D.; Kim, D.S.; Yoon, S.-S.; Koh, Y. Redefining the role of etoposide in first-line treatment of peripheral T-cell lymphoma. Blood Adv. 2017, 1, 2138–2146.
- Advani, R.; Horwitz, S.; Zelenetz, A.; Horning, S.J. Angioimmunoblastic T cell lymphoma: Treatment experience with cyclosporine. Leuk. Lymphoma 2007, 48, 521–525.
- Zhang, Y.; Lee, D.; Brimer, T.; Hussaini, M.; Sokol, L. Genomics of Peripheral T-Cell Lymphoma and Its Implications for Personalized Medicine. Front. Oncol. 2020, 10, 898.
- Horwitz, S.M.; Koch, R.; Porcu, P.; Oki, Y.; Moskowitz, A.; Perez, M.; Myskowski, P.; Officer, A.; Jaffe, J.D.; Morrow, S.N.; et al. Activity of the PI3K-δ,γ inhibitor duvelisib in a phase 1 trial and preclinical models of T-cell lymphoma. Blood 2018, 131, 888–898.
- Wilcox, R.A.; Sun, D.X.; Novak, A.; Dogan, A.; Ansell, S.M.; Feldman, A.L. Inhibition of Syk protein tyrosine kinase induces apoptosis and blocks proliferation in T-cell non-Hodgkin’s lymphoma cell lines. Leukemia 2009, 24, 229–232.
- Fiore, D.; Cappelli, L.V.; Broccoli, A.; Zinzani, P.L.; Chan, W.C.; Inghirami, G. Peripheral T cell lymphomas: From the bench to the clinic. Nat. Rev. Cancer 2020, 20, 323–342.
- Mossé, Y.P.; Voss, S.D.; Lim, M.; Rolland, D.; Minard, C.G.; Fox, E.; Adamson, P.; Wilner, K.; Blaney, S.M.; Weigel, B.J. Targeting ALK With Crizotinib in Pediatric Anaplastic Large Cell Lymphoma and Inflammatory Myofibroblastic Tumor: A Children’s Oncology Group Study. J. Clin. Oncol. 2017, 35, 3215–3221.
- Laimer, D.; Dolznig, H.; Kollmann, K.; Vesely, P.W.; Schlederer, M.; Merkel, O.; Schiefer, A.-I.; Hassler, M.R.; Heider, S.; Amenitsch, L.; et al. PDGFR blockade is a rational and effective therapy for NPM-ALK–driven lymphomas. Nat. Med. 2012, 18, 1699–1704.
- FDA Approves Crizotinib for Children and Young Adults with Relapsed or Refractory, Systemic Anaplastic Large Cell Lymphoma. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-crizotinib-children-and-young-adults-relapsed-or-refractory-systemic-anaplastic-large (accessed on 24 May 2021).
- Ishii, T.; Ishida, T.; Utsunomiya, A.; Inagaki, A.; Yano, H.; Komatsu, H.; Iida, S.; Imada, K.; Uchiyama, T.; Akinaga, S.; et al. Defucosylated Humanized Anti-CCR4 Monoclonal Antibody KW-0761 as a Novel Immunotherapeutic Agent for Adult T-cell Leukemia/Lymphoma. Clin. Cancer Res. 2010, 16, 1520–1531.
- Ishida, T.; Joh, T.; Uike, N.; Yamamoto, K.; Utsunomiya, A.; Yoshida, S.; Saburi, Y.; Miyamoto, T.; Takemoto, S.; Suzushima, H.; et al. Defucosylated Anti-CCR4 Monoclonal Antibody (KW-0761) for Relapsed Adult T-Cell Leukemia-Lymphoma: A Multicenter Phase II Study. J. Clin. Oncol. 2012, 30, 837–842.
- Flynn, M.J.; Zammarchi, F.; Tyrer, P.C.; Akarca, A.U.; Janghra, N.; Britten, C.E.; Havenith, C.E.; Levy, J.-N.; Tiberghien, A.; Masterson, L.A.; et al. ADCT-301, a Pyrrolobenzodiazepine (PBD) Dimer–Containing Antibody–Drug Conjugate (ADC) Targeting CD25-Expressing Hematological Malignancies. Mol. Cancer Ther. 2016, 15, 2709–2721.
- Collins, M.B.G.P.; Horwitz, S.M.; Davies, A.; Karnad, A.; Samaniego, F.; Spira, A.I.; A Fields, P.; Menne, T.; Boni, J.; Cruz, H.; et al. Adct-301 (Camidanlumab Tesirine), a Novel Pyrrolobenzodiazepine-Based CD25-Targeting Antibody Drug Conjugate, in a Phase 1 Study of Relapsed/Refractory Non-Hodgkin Lymphoma Shows Activity in T-Cell Lymphoma. Blood 2018, 132, 1658.
- Altmann, B.; Wulf, G.; Truemper, L.; D’Amore, F.; Relander, T.; Toldbod, H.; Delabie, J.M.; Rosenwald, A.; Ziepert, M.; Loeffler, M. Alemtuzumab Added to CHOP for Treatment of Peripheral T-Cell Lymphoma (PTCL) in Previously Untreated Young and Elderly Patients: Pooled Analysis of the International ACT-1/2 Phase III Trials. Blood 2018, 132, 1622.
- Morschhauser, F.; Fitoussi, O.; Haioun, C.; Thieblemont, C.; Quach, H.; Delarue, R.; Glaisner, S.; Gabarre, J.; Bosly, A.; Lister, J.; et al. A phase 2, multicentre, single-arm, open-label study to evaluate the safety and efficacy of single-agent lenalidomide (Revlimid®) in subjects with relapsed or refractory peripheral T-cell non-Hodgkin lymphoma: The EXPECT trial. Eur. J. Cancer 2013, 49, 2869–2876.
- Nolan, O.; O’Kennedy, R. Bifunctional antibodies: Concept, production and applications. Biochim. Biophys. Acta BBA Protein Struct. Mol. Enzym. 1990, 1040, 1–11.
- Ellerman, D. Bispecific T-cell engagers: Towards understanding variables influencing the in vitro potency and tumor selectivity and their modulation to enhance their efficacy and safety. Methods 2018, 154, 102–117.
- Goebeler, M.-E.; Knop, S.; Viardot, A.; Kufer, P.; Topp, M.S.; Einsele, H.; Noppeney, R.; Hess, G.; Kallert, S.; Mackensen, A.; et al. Bispecific T-Cell Engager (BiTE) Antibody Construct Blinatumomab for the Treatment of Patients With Relapsed/Refractory Non-Hodgkin Lymphoma: Final Results From a Phase I Study. J. Clin. Oncol. 2016, 34, 1104–1111.
- Constantinidou, A.; Alifieris, K.; Trafalis, D.T. Targeting Programmed Cell Death -1 (PD-1) and Ligand (PD-L1): A new era in cancer active immunotherapy. Pharmacol. Ther. 2018, 194, 84–106.
- Kwong, Y.-L.; Chan, T.S.Y.; Tan, D.; Kim, S.J.; Poon, L.-M.; Mow, B.; Khong, P.-L.; Loong, F.; Au-Yeung, R.; Iqbal, J.; et al. PD1 blockade with pembrolizumab is highly effective in relapsed or refractory NK/T-cell lymphoma failing l-asparaginase. Blood 2017, 129, 2437–2442.
- Ng, S.Y.; Jacobsen, E.D. Peripheral T-Cell Lymphoma. Hematol. Clin. N. Am. 2019, 33, 657–668.
- Ratner, L.; Waldmann, T.A.; Janakiram, M.; Brammer, J.E. Rapid Progression of Adult T-Cell Leukemia–Lymphoma after PD-1 Inhibitor Therapy. N. Engl. J. Med. 2018, 378, 1947–1948.
- Anand, K.; Ensor, J.; Pingali, S.R.; Hwu, P.; Duvic, M.; Chiang, S.; Miranda, R.; Zu, Y.; Iyer, S. T-cell lymphoma secondary to checkpoint inhibitor therapy. J. Immunother. Cancer 2019, 8, e000104.
- Ward, G.A.; Ahn, J.S.; Lyons, J.F.; Martins, V.; Smyth, T.; Chessari, G.; Lewis, E.J.; Johnson, C.N.; Munck, J.M.; Rich, S.J.; et al. ASTX660, a Novel Non-peptidomimetic Antagonist of cIAP1/2 and XIAP, Potently Induces TNFα-Dependent Apoptosis in Cancer Cell Lines and Inhibits Tumor Growth. Mol. Cancer Ther. 2018, 17, 1381–1391.
- Dubrez, L.; Berthelet, J.; Glorian, V. IAP proteins as targets for drug development in oncology. OncoTargets Ther. 2013, 9, 1285–1304.
- Fulda, S. Inhibitor of Apoptosis (IAP) proteins in hematological malignancies: Molecular mechanisms and therapeutic opportunities. Leukemia 2014, 28, 1414–1422.
- Mita, M.M.; Lorusso, P.M.; Papadopoulos, K.P.; Gordon, M.S.; Mita, A.C.; Ferraldeschi, R.; Keer, H.; Oganesian, A.; Su, X.Y.; Jueliger, S.; et al. A Phase I Study of ASTX660, an Antagonist of Inhibitors of Apoptosis Proteins, in Adults with Advanced Cancers or Lymphoma. Clin. Cancer Res. 2020, 26, 2819–2826.
- Samaniego, F.; Hollebecque, A.; Foss, F.; Lister, J.; Mita, M.; Wagner-Johnston, N.; Dyer, M.; You, B.; Prica, A.; Hernandez-Llizaliturri, F.; et al. Preliminary results of astx660, a novel non-peptidomimetic ciap1/2 and xiap antagonist, in relapsed/refractory peripheral t-cell lymphoma and cutaneous T cell lymphoma. Hematol. Oncol. 2019, 37, 527.
- Savoldo, B.; Rooney, C.M.; Di Stasi, A.; Abken, H.; Hombach, A.; Foster, A.E.; Zhang, L.; Heslop, H.E.; Brenner, M.K.; Dotti, G. Epstein Barr virus–specific cytotoxic T lymphocytes expressing the anti-CD30ζ artificial chimeric T-cell receptor for immunotherapy of Hodgkin disease. Blood 2007, 110, 2620–2630.
- Wang, C.-M.; Wu, Z.-Q.; Wang, Y.; Guo, Y.-L.; Dai, H.-R.; Wang, X.-H.; Li, X.; Zhang, Y.-J.; Zhang, W.-Y.; Chen, M.-X.; et al. Autologous T Cells Expressing CD30 Chimeric Antigen Receptors for Relapsed or Refractory Hodgkin Lymphoma: An Open-Label Phase I Trial. Clin. Cancer Res. 2016, 23, 1156–1166.
- Ramos, C.A.; Ballard, B.; Zhang, H.; Dakhova, O.; Gee, A.P.; Mei, Z.; Bilgi, M.; Wu, M.-F.; Liu, H.; Grilley, B.; et al. Clinical and immunological responses after CD30-specific chimeric antigen receptor–redirected lymphocytes. J. Clin. Investig. 2017, 127, 3462–3471.
- Maciocia, P.; Wawrzyniecka, P.A.; Philip, B.; Ricciardelli, I.; Akarca, A.U.; Onuoha, S.C.; Legut, M.; Cole, D.; Sewell, A.K.; Gritti, G.; et al. Targeting the T cell receptor β-chain constant region for immunotherapy of T cell malignancies. Nat. Med. 2017, 23, 1416–1423.
- Rezvani, K.; Rouce, R.; Liu, E.; Shpall, E. Engineering Natural Killer Cells for Cancer Immunotherapy. Mol. Ther. 2017, 25, 1769–1781.
This entry is offline, you can click here to edit this entry!
