Chemical looping technology in general, is the rising star in chemical technologies, which is capable of low CO2 emissions with applications in the production of heat, fuels, chemicals, and electricity. This entry discusses the technology in general, gives an overview of some pilot scale plants and the different chemical looping processes with focus on the production of heat and chemicals, highlights the importance of the development of oxygen carrier materials with suitable properties,
- chemical looping
- oxygen carrier
- hydrogen
- CO2 utilization
- metal oxide
- carbon capture and storage
- reforming
- fossil fuels
1. Introduction
Carbon dioxide emissions resulting from combustion and other chemical processes gravely impact the environment. Therefore, alternative processes have been developed in which these CO2 emissions can be avoided. Among current and emerging technologies for CO2 capture, chemical looping combustion (CLC) was frequently mentioned as a particularly promising approach to combining CO2 capture and energy production[1][2][3]. This technology can also be included in the oxyfuel combustion branch of CCS, as in this process also, all diluting components of the air are separated before the combustion of the fuel. The main difference with conventional oxy-fuel capture is the avoidance of a separate costly air separation unit. Pure oxygen is separated from the air inside the chemical looping process itself, by the utilization of metal oxides, which selectively transfer oxygen from the air to the fuel. These oxygen transfer materials are hence commonly called ‘oxygen carriers’ (OC). CLC has gained significant maturity during the last decades, resulting in kWth and MWth-scale operation at various locations throughout the world (see Table 1). Chemical looping technology in general, is the rising star in chemical technologies, which is capable of low CO2 emissions with applications in the production of heat, fuels, chemicals, and electricity. Several aspects are being considered in the current transition and scale-up of the technology to a
level appropriate for industrial implementation. One key critical aspect is the presence of a suitable, sustainable, and cost-e ective oxygen carrier material with the right properties for the specific chemical looping application.
The origin of chemical looping technology is said to start way back in the year 1950 when Lewis and Gilliland filed their patent entitled ”production of pure carbon dioxide”[4]. In this patent, an oxidizable carbonaceous material was oxidized by copper oxide particles to produce carbon dioxide free of inert gases, such as nitrogen. The term ‘oxygen carrier’, which is still used to denote the solid materials that transfer the oxygen from oxidizing agent to fuel, was already introduced then[4]. The term ‘chemical looping’ was derived by Ishida et al. in the second half of the 1980s from the different oxidation and reduction reactions through the oxygen carrier loops, yielding net combustion of the fuel[5]. Since then, the technology has matured significantly. The process has been scaled up to MWth-scale, more than 600 oxygen carrier materials have been developed, and these research activities have resulted in a few thousand publications and several review papers across all domains ranging from reactor design, oxygen carrier design for both combustion and chemicals production to scale up, and operational experience in the units across the world[6][7][8][9][10][11][12][13][14][15][16][17][18][19][20][21][22][23][24][25][26][27]. Five international conferences dedicated specifically to chemical looping have been organized during the past 10 years all over the world.
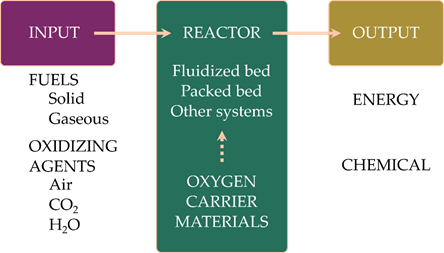
Figure 1 gives an overview of all relevant aspects of chemical looping. Different types of carbonaceous fuels are used, depending on the focus of the process. While solid fuels are mainly used for energy production, gaseous fuels are interesting for the production of chemicals. In chemical looping processes air is used for replenishing the oxygen in the oxygen carriers. In addition to air, carbon dioxide and steam can also be used. In this way pure CO and H2 can be generated. As the reactions between the oxygen carrier and CO2 or H2O are endothermic, changing the oxidizing agent in the chemical looping process has a large impact on the energy balances of the process. Different reactor systems can be used in chemical looping processes, ranging from packed-bed reactors to interconnected fluidized-bed systems. Each reactor concept has advantages and disadvantages, and their suitability depends also on the reactivity, composition and the properties of the oxygen carrier materials[6].
Table 2. Select fluidized bed chemical looping process development and pilot plants across the world, adapted from [28].
|
Institution |
Location |
Year |
Capacity (kWth) |
Ref |
|
Vienna University of Technology |
Vienna, Austria |
2009 |
120 |
[29] |
|
Hamburg University of Technology |
Hamburg, Germany |
2012 |
25 |
[30] |
|
Chalmers University of Technology |
Gothenburg, Sweden |
2012 |
100 |
[31] |
|
Darmstadt University of Technology |
Darmstadt, Germany |
2012 |
1000 |
[32] |
|
Southeast University |
Nanjing, China |
2012 |
50 |
[33] |
|
University of Utah |
Salt Lake City, USA |
2012 |
200 |
[28] |
|
National Energy Technology Laboratory |
Morgantown, USA |
2013 |
50 |
[34] |
|
Instituto de Carboquímica (ICB-CSIC) |
Zaragoza, Spain |
2014 |
50 |
[35] |
|
Huazhong University of Sci. and Tech. |
Wuhan, China |
2016 |
50 |
[36] |
|
VTT Technical Research Center |
Espoo, Finland |
2016 |
50 |
[37] |
|
Japan Coal Energy Center |
Tokyo, Japan |
2017 |
100 |
[38] |
|
Korean Institute of Energy Research |
Daejeon, Korea |
2018 |
500 |
[39] |
Figure 1. A general overview of the parts of the chemical looping process.
2. Oxygen Carrier Materials
A key issue in the further development of this rising star in chemical technologies and its introduction to the industry is the selection and further development of an appropriate oxygen carrier (OC) material [6]. This solid oxygen carrier material supplies the stoichiometric oxygen needed for the various chemical processes. Its reactivity, cost, toxicity, thermal stability, attrition resistance, and chemical stability are critical selection criteria for developing suitable oxygen carrier materials[40]. To develop oxygen carriers with optimal properties and long-term stability, one must consider the employed reactor configuration and the aim of the chemical looping process, as well as the thermodynamic properties of the active phases, their interaction with the used support material [41][42], long-term stability, internal ionic migration[6], and the advantages and limits of the employed synthesis methods[6].
3. Different Focus of Chemical Looping Processes
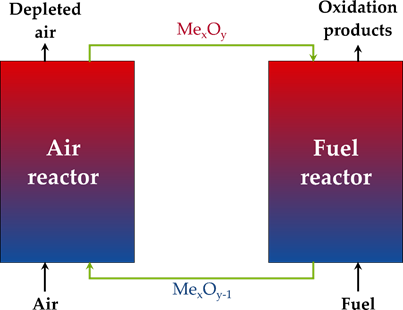
Commonly, chemical looping is used to denote cyclic processes that use a solid material, which circulates the oxygen required for the conversion of a fuel. This solid material is hence called ‘oxygen carrier’ and consists traditionally of metal oxide particles. To close the chemical loop, the oxygen-depleted solid oxygen carrier must be re-oxidized before starting a new cycle conventionally with the use of air. When the goal of the process is energy production, the fuel is converted to total oxidation products (CO2 and H2O), and the oxygen-depleted solid must be regenerated by the O2 in air. The process is then known as chemical looping combustion (CLC) (See Figure 2 for a generalized process scheme). The large advantage of CLC is the inherent separation of the N2 from the oxidizing air and the produced CO2 in the process. The H2O that is still present in the flue gas can easily be condensed, and a pure CO2 stream is obtained without additional separation costs, such as needed in post-combustion CCS[18]. There is also no need for an expensive air separation unit, such as used in conventional oxy-fuel combustion[43].
Figure 2. General flowsheet of the chemical looping process.
When chemical looping technology started to gain more attention from researchers, it was predominantly being developed for efficient combustion of fuels, such as coal or natural gas. This technology is commonly called chemical looping combustion, and it is currently, after several decades of research, gaining maturity during the pilot and semi-industrial scale[28].
More recently, however, the focus of chemical looping is shifting more towards the production of hydrogen and other chemicals instead of energy [22][44][45][46][47][47][48]. In this way, products with more economic added value can be generated, which increase the economic viability of the technology in the current context, even at smaller scales. This is expected to facilitate the introduction of the technology into industry. When the focus of the process shifts more towards the production of chemicals, oxygen carrier materials are regenerated by other oxidizing agents instead of air, such as CO2 [49][50][51][52] or even H2O [26][49][53][54], with respective productions of CO and H2. Some of the different chemical looping technologies, which can be found in the literature, are included in Table 2, as well as the used primary fuels, the abbreviation of the CL-branch technology, and the reactor types used. Fuels that are used in chemical looping include coal, liquid fuels, biomass, and natural gas[6]. For characterization purposes, sometimes, syngas or hydrogen is used, but these are not used at an industrial scale[6]. While the initial focus of CLC was the combustion of gaseous fuels, now, gaseous fuels are predominantly converted to chemicals, while combustion for energy production is more frequently executed with solid-fuels (see Table 2). There is, however, some overlap.
Table 2. An overview of some of the different chemical looping technologies found in the literature.
|
Focus |
Primary Fuel |
Process |
Reactor Type |
|
|
Combustion |
Gas |
CLC |
Chemical Looping Combustion |
f1, m2, p3 |
|
- |
Solid |
Syngas-CLC |
Syngas-Chemical Looping Combustion |
f, m, p |
|
- |
Solid |
iG-CLC |
In situ Gasification Chemical Looping Combustion |
f, m |
|
- |
Solid |
CLOU |
Chemical Looping with Oxygen Uncoupling |
f |
|
- |
Gas |
GSC |
Gas Switching Combustion |
f |
|
Chemicals |
Gas |
SR-CLR |
Steam Reforming-Chemical Looping Reforming |
f |
|
- |
Gas, liquid |
a-CLR |
Autothermal-Chemical Looping Reforming |
f |
|
- |
Gas |
GSR |
Gas Switching Reforming |
f |
|
- |
Gas |
CSR |
Chemical Switching Reforming |
f |
|
- |
Gas, liquid |
SE-CLSR |
Sorption Enhanced-Chemical Looping Steam Reforming |
f, m, p |
|
- |
Gas |
CLHG/TRCH/OSD |
Chemical Looping for Hydrogen Generation/Three Reactor Chemical Looping/One Step Decarbonization |
f, m, p |
|
- |
Solid |
SCL |
Syngas Chemical Looping |
m |
|
- |
Solid |
CDCL |
Coal Direct Chemical Looping |
f, m |
|
Oxygen Production |
/ |
CLAS |
Chemical Looping Air Separation |
f, m, p |
4. Outlook
The authors expect that the interaction between catalysis and chemical looping will make significant changes to the chemical looping landscape in the following years and, because of its potential benefits,
deserves extensive attention from the research community.
This entry is adapted from the peer-reviewed paper 10.3390/catal10080926
References
- Calin-Cristian Cormos; Evaluation of syngas-based chemical looping applications for hydrogen and power co-generation with CCS. International Journal of Hydrogen Energy 2012, 37, 13371-13386, 10.1016/j.ijhydene.2012.06.090.
- S. Sengodan; Rong Lan; John Humphreys; Dongwei Du; Wei Xu; Huanting Wang; Shanwen Tao; Advances in reforming and partial oxidation of hydrocarbons for hydrogen production and fuel cell applications. Renewable and Sustainable Energy Reviews 2018, 82, 761-780, 10.1016/j.rser.2017.09.071.
- Lin Zhu; Yangdong He; Luling Li; Pengbin Wu; Tech-economic assessment of second-generation CCS: Chemical looping combustion. Energy 2018, 144, 915-927, 10.1016/j.energy.2017.12.047.
- Gilliland, R.E.; Lewis, W.K. Production of Pure Carbon Dioxide. U.S. Patent 2,665,972, 13 November 1950.
- M. Ishida; D. Zheng; T. Akehata; Evaluation of a chemical-looping-combustion power-generation system by graphic exergy analysis. Energy 1987, 12, 147-154, 10.1016/0360-5442(87)90119-8.
- De Vos Yoran, Jacobs Marijke, Van Der Voort Pascal, Van Driessche Isabel, Snijkers Frans and Verberckmoes An; Development of Stable Oxygen Carrier Materials for Chemical Looping Processes—A Review. Catalysts 2020, 10(8), 926, 10.3390/catal10080926.
- Juan Adanez; Alberto Abad; Francisco García-Labiano; Pilar Gayán; Luis F. De Diego; Progress in Chemical-Looping Combustion and Reforming technologies. Progress in Energy and Combustion Science 2012, 38, 215-282, 10.1016/j.pecs.2011.09.001.
- Matthew E Boot-Handford; Juan Carlos Abanades; Edward Anthony; Martin J. Blunt; Stefano Brandani; Niall Mac Dowell; José R. Fernández; Maria-Chiara Ferrari; Robert Gross; Jason P. Hallett; et al. Carbon capture and storage update. Energy & Environmental Science 2014, 7, 130-189, 10.1039/c3ee42350f.
- He Fang; Li Haibin; Zhao Zengli; Advancements in Development of Chemical-Looping Combustion: A Review. International Journal of Chemical Engineering 2009, 2009, 1-16, 10.1155/2009/710515.
- Juan Carlos Abanades; Borja Arias; Anders Lyngfelt; Tobias Mattisson; D.E. Wiley; H. Li; Minh T. Ho; Enzo Mangano; Stefano Brandani; Emerging CO2 capture systems. International Journal of Greenhouse Gas Control 2015, 40, 126-166, 10.1016/j.ijggc.2015.04.018.
- Anirban Nandy; Chanchal Loha; Sai Gu; Pinaki Sarkar; Malay K. Karmakar; Pradip K. Chatterjee; Present status and overview of Chemical Looping Combustion technology. Renewable and Sustainable Energy Reviews 2016, 59, 597-619, 10.1016/j.rser.2016.01.003.
- Mohammad M. Hossain; Hugo I. De Lasa; Chemical-looping combustion (CLC) for inherent CO2 separations—a review. Chemical Engineering Science 2008, 63, 4433-4451, 10.1016/j.ces.2008.05.028.
- J. Adánez; A. Abad; T. Mendiara; Pilar Gayán; Luis F. De Diego; F. García-Labiano; Chemical looping combustion of solid fuels. Progress in Energy and Combustion Science 2018, 65, 6-66, 10.1016/j.pecs.2017.07.005.
- Anders Lyngfelt; Chemical-looping combustion of solid fuels – Status of development. Applied Energy 2014, 113, 1869-1873, 10.1016/j.apenergy.2013.05.043.
- Anders Lyngfelt; Carl Linderholm; Chemical-Looping Combustion of Solid Fuels – Status and Recent Progress. Energy Procedia 2017, 114, 371-386, 10.1016/j.egypro.2017.03.1179.
- Ping Wang; Nicholas Means; Dushyant Shekhawat; David A Berry; Mehrdad Massoudi; Chemical-Looping Combustion and Gasification of Coals and Oxygen Carrier Development: A Brief Review. Energies 2015, 8, 10605-10635, 10.3390/en81010605.
- Anders Lyngfelt; Oxygen Carriers for Chemical Looping Combustion - 4 000 h of Operational Experience. Oil & Gas Science and Technology – Revue d’IFP Energies nouvelles 2011, 66, 161-172, 10.2516/ogst/2010038.
- Behdad Moghtaderi; Review of the Recent Chemical Looping Process Developments for Novel Energy and Fuel Applications. Energy & Fuels 2011, 26, 15-40, 10.1021/ef201303d.
- Tao Song; Laihong Shen; Review of reactor for chemical looping combustion of solid fuels. International Journal of Greenhouse Gas Control 2018, 76, 92-110, 10.1016/j.ijggc.2018.06.004.
- Amit Mishra; Fanxing Li; Chemical looping at the nanoscale — challenges and opportunities. Current Opinion in Chemical Engineering 2018, 20, 143-150, 10.1016/j.coche.2018.05.001.
- Tobias Mattisson; Martin Keller; Carl Linderholm; Patrick Moldenhauer; Magnus Rydén; Henrik Leion; Anders Lyngfelt; Chemical-looping technologies using circulating fluidized bed systems: Status of development. Fuel Processing Technology 2018, 172, 1-12, 10.1016/j.fuproc.2017.11.016.
- Ming Luo; Yang Yi; Shuzhong Wang; Zhuliang Wang; Min Du; Jianfeng Pan; Qian Wang; Review of hydrogen production using chemical-looping technology. Renewable and Sustainable Energy Reviews 2018, 81, 3186-3214, 10.1016/j.rser.2017.07.007.
- Zhuo Cheng; Lang Qin; Jonathan A. Fan; Liang-Shih Fan; New Insight into the Development of Oxygen Carrier Materials for Chemical Looping Systems. Engineering 2018, 4, 343-351, 10.1016/j.eng.2018.05.002.
- Michael Matzen; Jessica Pinkerton; Xiaomeng Wang; Yaşar Demirel; Use of natural ores as oxygen carriers in chemical looping combustion: A review. International Journal of Greenhouse Gas Control 2017, 65, 1-14, 10.1016/j.ijggc.2017.08.008.
- Vidya S Batra; Hung-Pin Li; Oxygen carrier materials and their role in chemical looping reactions for fuel conversion. Current Opinion in Chemical Engineering 2017, 15, 44-48, 10.1016/j.coche.2016.11.006.
- L. N. Protasova; Frans Snijkers; Recent developments in oxygen carrier materials for hydrogen production via chemical looping processes. Fuel 2016, 181, 75-93, 10.1016/j.fuel.2016.04.110.
- Jakkapong Udomsirichakorn; P. Abdul Salam; Review of hydrogen-enriched gas production from steam gasification of biomass: The prospect of CaO-based chemical looping gasification. Renewable and Sustainable Energy Reviews 2014, 30, 565-579, 10.1016/j.rser.2013.10.013.
- Whitty, K.; Wagner, D.R.; Backman, M.; Dobo, Z.; Merrett, K.M.; Dai, J. Experience with Chemical LoopingCombustion of Coal in a 200 kWth Dual Fluidized Bed Reactor. In Proceedings of the 5th Internationalconference on Chemical Looping, Park City, UT, USA, 24–27 September 2018; p. 18
- Tobias Pröll; Philipp Kolbitsch; Johannes Bolhàr-Nordenkampf; Hermann Hofbauer; A novel dual circulating fluidized bed system for chemical looping processes. AIChE Journal 2009, 55, 3255-3266, 10.1002/aic.11934.
- Johannes Haus; Kai Lyu; Ernst-Ulrich Hartge; Stefan Heinrich; Joachim Werther; Analysis of a Two-Stage Fuel Reactor System for the Chemical-Looping Combustion of Lignite and Bituminous Coal. Energy Technology 2016, 4, 1263-1273, 10.1002/ente.201600102.
- Pontus Markström; Carl Linderholm; Anders Lyngfelt; Chemical-looping combustion of solid fuels – Design and operation of a 100kW unit with bituminous coal. International Journal of Greenhouse Gas Control 2013, 15, 150-162, 10.1016/j.ijggc.2013.01.048.
- Jochen Ströhle; Matthias Orth; Bernd Epple; Design and operation of a 1 MWth chemical looping plant. Applied Energy 2014, 113, 1490-1495, 10.1016/j.apenergy.2013.09.008.
- Rui Xiao; Liangyong Chen; Chiranjib Saha; Shuai Zhang; Sankar Bhattacharya; Pressurized chemical-looping combustion of coal using an iron ore as oxygen carrier in a pilot-scale unit. International Journal of Greenhouse Gas Control 2012, 10, 363-373, 10.1016/j.ijggc.2012.07.008.
- Weber, J.; Straub, D.; Breault, R.W.; Richards, G. Operating Experience of a Chemical Looping CirculatingFluidized Bed Combustor. In Proceedings of the 39th International Technical Conference on Clean Coal &Fuel Systems, Clearwater, FL, USA, 1–5 June 2014.
- Alberto Abad; Raul Perez-Vega; Luis F. De Diego; Francisco García-Labiano; Pilar Gayán; Juan Adanez; Design and operation of a 50 kWth Chemical Looping Combustion (CLC) unit for solid fuels. Applied Energy 2015, 157, 295-303, 10.1016/j.apenergy.2015.03.094.
- Ma, J.; Zhao, H.; Niu, P.; Chen, X.; Tian, X.; Zheng, C. Design and Operation of a 50 kWth Chemical LoopingCombustion (CLC) Reactor using Coal as Fuel. In Proceedings of the 4th International Conference onChemical Looping, Southeast University, Nanjing, China, 26–28 September 2016.
- Pikkarainen, T.; Hiltunen, I.; Tier, S. Piloting of Bio-CLC for BECCS. In Proceedings of the 4th InternationalConference on Chemical Looping, Nanjing, China, 26–28 September 2016.
- Lin, S.Y.; Saito, T. Development of Three-Tower (Reactors) Technology for Chemical Looping CoalCombustion. In Proceedings of the 4th International Conference on Chemical Looping, Nanjing, China,26–28 September 2016.
- Hojung Ryu; Ngho Lee; Myoungsoo Jang; Junghwan Kim; Jeom-In Baek; Conceptual Design and Feasibility Study on 0.5 MWth Pressurized Chemical Looping Combustor. Transactions of the Korean hydrogen and new energy society 2016, 27, 201-210, 10.7316/khnes.2016.27.2.201.
- Yoran De Vos; Marijke Jacobs; Pascal Van Der Voort; Isabel Van Driessche; F. M. M. Snijkers; An Verberckmoes; Optimization of spray dried attrition-resistant iron based oxygen carriers for chemical looping reforming. Chemical Engineering Journal 2017, 309, 824-839, 10.1016/j.cej.2016.10.092.
- Yoran De Vos; Marijke Jacobs; Pascal Van Der Voort; Isabel Van Driessche; F. M. M. Snijkers; An Verberckmoes; Sustainable iron-based oxygen carriers for Chemical Looping for Hydrogen Generation. International Journal of Hydrogen Energy 2019, 44, 1374-1391, 10.1016/j.ijhydene.2018.11.099.
- Yoran De Vos; Antonis Vamvakeros; Dorota Matras; Marijke Jacobs; Pascal Van Der Voort; Isabel Van Driessche; Simon Jacques; Vesna Middelkoop; An Verberckmoes; Sustainable iron-based oxygen carriers for hydrogen production – Real-time operando investigation. International Journal of Greenhouse Gas Control 2019, 88, 393-402, 10.1016/j.ijggc.2019.06.016.
- Giuseppe Diglio; Piero Bareschino; Erasmo Mancusi; Francesco Pepe; Novel quasi-autothermal hydrogen production process in a fixed-bed using a chemical looping approach: A numerical study. International Journal of Hydrogen Energy 2017, 42, 15010-15023, 10.1016/j.ijhydene.2017.05.017.
- Samira Parishan; Patrick Littlewood; Aleks Arinchtein; Vinzenz Fleischer; Reinhard Schomäcker; Chemical looping as a reactor concept for the oxidative coupling of methane over the MnxOy-Na2WO4/SiO2 catalyst, benefits and limitation. Catalysis Today 2018, 311, 40-47, 10.1016/j.cattod.2017.08.019.
- Vinzenz Fleischer; Patrick Littlewood; Samira Parishan; Reinhard Schomäcker; Reinhard Schomäcker; Chemical looping as reactor concept for the oxidative coupling of methane over a Na 2 WO 4 /Mn/SiO 2 catalyst. Chemical Engineering Journal 2016, 306, 646-654, 10.1016/j.cej.2016.07.094.
- Jing Chen; Kun Zhao; Zengli Zhao; Fang He; Zhen Huang; Guoqiang Wei; Changrong Xia; Reaction schemes of barium ferrite in biomass chemical looping gasification for hydrogen-enriched syngas generation via an outer-inner looping redox reaction mechanism. Energy Conversion and Management 2019, 189, 81-90, 10.1016/j.enconman.2019.03.084.
- Seyyed Yaghoob Hosseini; M.R. Khosravi Nikou; Ahmad Shariati; Production of hydrogen and syngas using chemical looping technology via cerium-iron mixed oxides. Chemical Engineering and Processing - Process Intensification 2019, 139, 23-33, 10.1016/j.cep.2019.03.018.
- Yajing Wang; Yane Zheng; Yuhao Wang; Kongzhai Li; Yaming Wang; Lihong Jiang; Xing Zhu; Yonggang Wei; Hua Wang; Syngas production modified by oxygen vacancies over CeO2-ZrO2-CuO oxygen carrier via chemical looping reforming of methane. Applied Surface Science 2019, 481, 151-160, 10.1016/j.apsusc.2019.03.050.
- Mingchen Tang; Long Xu; Maohong Fan; Progress in oxygen carrier development of methane-based chemical-looping reforming: A review. Applied Energy 2015, 151, 143-156, 10.1016/j.apenergy.2015.04.017.
- Mohammad Ismail; Wen Liu; Matthew T Dunstan; Stuart A. Scott; Development and performance of iron based oxygen carriers containing calcium ferrites for chemical looping combustion and production of hydrogen. International Journal of Hydrogen Energy 2016, 41, 4073-4084, 10.1016/j.ijhydene.2015.11.066.
- Vladimir Galvita; Hilde Poelman; Christophe Detavernier; Guy B. Marin; Catalyst-assisted chemical looping for CO2 conversion to CO. Applied Catalysis B: Environmental 2015, 164, 184-191, 10.1016/j.apcatb.2014.09.007.
- Marcus Wenzel; N.V.R. Aditya Dharanipragada; Vladimir Galvita; Hilde Poelman; Guy B. Marin; Liisa K. Rihko-Struckmann; Kai Sundmacher; CO production from CO 2 via reverse water–gas shift reaction performed in a chemical looping mode: Kinetics on modified iron oxide. Journal of CO2 Utilization 2017, 17, 60-68, 10.1016/j.jcou.2016.10.015.
- Martin S. C. Chan; Wen Liu; Mohammad Ismail; Yanhui Yang; Stuart A. Scott; John S. Dennis; Improving hydrogen yields, and hydrogen:steam ratio in the chemical looping production of hydrogen using Ca2Fe2O5. Chemical Engineering Journal 2016, 296, 406-411, 10.1016/j.cej.2016.03.132.
- Yoran De Vos; Marijke Jacobs; Isabel Van Driessche; Pascal Van Der Voort; Frans Snijkers; An Verberckmoes; Processing and characterization of Fe-based oxygen carriers for chemical looping for hydrogen production. International Journal of Greenhouse Gas Control 2018, 70, 12-21, 10.1016/j.ijggc.2018.01.007.
- Vladimir Galvita; Hilde Poelman; Guy B Marin; Hydrogen Production from Methane and Carbon Dioxide by Catalyst-Assisted Chemical Looping. Topics in Catalysis 2011, 54, 907-913, 10.1007/s11244-011-9709-7.
- N.V.R. Aditya Dharanipragada; Vladimir Galvita; Hilde Poelman; Lukas C. Buelens; Christophe Detavernier; Guy B. Marin; Bifunctional Co- and Ni- ferrites for catalyst-assisted chemical looping with alcohols. Applied Catalysis B: Environmental 2018, 222, 59-72, 10.1016/j.apcatb.2017.09.067.
- N.V.R. Aditya Dharanipragada; Vladimir Galvita; Hilde Poelman; Lukas C. Buelens; Christophe Detavernier; Guy B. Marin; Bifunctional Co- and Ni- ferrites for catalyst-assisted chemical looping with alcohols. Applied Catalysis B: Environmental 2018, 222, 59-72, 10.1016/j.apcatb.2017.09.067.