Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biochemical Research Methods
Cocona fruits are a popular food and medicinal fruit used mainly in the Amazon and several countries of South America for the preparation of several food products such as drinks, jams and milk shakes. In this study five ecotypes of cocona native to Peru have been studied regarding their nutritional and antioxidants values plus antihyperlipidemic activities.
- antioxidant activity
- UHPLC-PDA-ESI-OT-MS
- Solanaceae
- nutritional values
- phenolics
- antihyperlipidemic
1. Introduction
In recent decades, global interest has increased in search of the chemical composition and biological activities of natural sources since many of the compounds present in biological sources such as local plants and marine organisms are important for the protection of human health. The fruit of cocona (Solanum sessiliflorum Dunal; Solanaceae) are native of the Amazonian tropic. Just like other plants in the genus Solanum, they exhibit a morphological diversity corresponding to the variability in habitat and ecology changes, as well as with the process of domestication of the species; However, little is known about its chemical composition and the implications of the terroir and climate in its morphology and nutrient and health beneficial properties. The common name of the fruit in Spanish or Portuguese speaking countries is cocona, topiro or cubiu, and is known as “Orinoco apple” and “peach tomato” in English speaking countries. It is an endemic species to Amazon cultivated by natives and settlers in agroforestry arrangements and chagras in their settlement sites which improves it on a traditional food from this area. The pulp is the edible part of the fruit and is known for its refreshing flavor to produce refreshments, milk shakes, jams, and jellies and for the medicinal properties, including amelioration of itching produced by insect bites, elimination of parasites, it is also use as topical to heal burns and for the control of cholesterol, diabetes, and uric acid. The studies carried out on the chemistry of the species are few and no complete, mainly limited to the report of some phenolic compounds and volatile metabolites which cannot depict the differentiation in the variations in the chemical composition between the different morphotypes of the fruit which is very important to give added values to the different products and ecotypes. This study aimed to differentiate five varieties of cocona (Figure 1) with the use of UHPLC coupled to high resolution mass spectrometry (UHPLC-PDA-ESI-OT-MS) grown in Peru, called oval, small round, large round big oval round and big square morphotypes, by means of the metabolite fingerprinting of the secondary metabolites present in mature fruits of the species. These adaptive morphotypes of the fruit depends directly on various biological factors and has been diversifying during the evolution and natural selection and can have the ability to produce different biologically important metabolites. Several carotenoids, alkaloids, organic acids, phenolic acids, flavonoid glycosides, coumarins, tannins, and volatile and fixed acids were reported to occur in fresh cocona fruits from Brazil [1][2] and recently, caffeoyl quinic acid was reported as the main important phenolic in the fruits [3], whereas determination of volatile organic compounds (VOCs) was done by HS-SPME/GC-MS in some ecotypes from Brazil [4], besides, extracts of those Brazilian ecotypes presented high concentrations of caffeic and gallic acids, beta-carotene, catechin, quercetin, and rutin and showed low density lipoproteins oxidation; cytotoxic and antiproliferative effect on breast (MCF-7) and colorectal (HT-29) cancer cell lines [5]. The purpose of this work is to contribute to the full phytochemical study of the Peruvian ecotype species through UHPLC-PDA-ESI-OT-MS for full untargeted metabolomic analyses to promote in fruit growers and fruit processors in the Peruvian Amazon the adoption of strategies for the sustainable use of the more promising ones based on its phenolic content and intrinsic health related properties, such as antihyperlipidemic capacities of the studied five ecotypes of this highly consumed fruit from Peru. Finally, proximal composition and mineral contents, plus the antioxidant activities thorough different methods and total carotene and phenolic contents of all ecotype pulps were tested and compared. The UHPLC full MS and PDA fingerprint analysis, was also performed for the ecotypes.

Figure 1. Pictures of cocona fruits NMA1, SRN9, CD1, CTR, UNT2 ecotypes.
2. Nutritional and Physicochemical Properties of 5 Cocona Ecotypes
Table 1 shows proximal composition such as the humidity, ashes, protein, lipids, carbohydrates, and fiber, while Table 2 shows the mineral contents of five ecotypes of cocona. Proximal composition of cocona fruits NMA1, SRN9, CD1, CTR, UNT2 ecotypes was performed. The results of physicochemical properties showed that the proximal composition and the caloric value of this fruit are similar to that showed for other species, but with a high fiber (from 1.08 to 1.93) and carbohydrate content (from 3.12–4.24). The edible portion of cocona ecotypes were analyzed for mineral content (Ca, Na, Mg, K, Cu, Mn, Zn, and Fe). The foods were generally high in K (570.83–2382.24 mg K/100 g edible portion) and low in sodium (3.25–6.87 mg Na/100 g edible portion). The five ecotypes had the highest contents in most of the elements, especially in calcium (17.85–70.07 mg Ca/100 g edible portion) and iron (52–71 mg Fe/100 g edible portion).
Table 1. Proximal composition of cocona fruits NMA1, SRN9, CD1, CTR, UNT2 ecotypes.
| Ecotypes | Humidity | Ashes | Total Lipids | Crude protein | Crude fiber | Carbohydrates |
|---|---|---|---|---|---|---|
| NMA1 | 91.85 ± 0.09 a | 0.75 ± 0.01 a | 0.65 ± 0.00 a | 1.08 ± 0.04 a | 1.68 ± 0.04 a | 3.99 |
| CD1 | 86.64 ± 0.36 b | 1.24 ± 0.06 b | 0.88 ± 0.00 b | 1.93 ± 0.05 b | 5.03 ± 0.15 b | 4.28 |
| CTR | 92.82 ± 0.03 c | 0.71 ± 0.03 a | 0.45 ± 0.01 c | 1.09 ± 0.04 a | 1.03 ± 0.05 c | 3.9 |
| SRN9 | 86.67 ± 0.12 b | 0.94 ± 0.02 c | 0.93 ± 0.01 d | 2.72 ± 0.04 c | 4.76 ± 0.17 b | 3.98 |
| UNT2 | 93.52 ± 0.08 d | 0.79 ± 0.02 a | 0.19 ± 0.00 e | 1.64 ± 0.06 d | 0.76 ± 0.03 c | 3.1 |
Each value represents the means ± SEM of three replicates, n = 3, while different letters on the same column indicate significant difference using Tukey test at 0.05 level of significance (p < 0.05).
Table 2. Mineral content (mg/100 g fresh pulp) of cocona fruits NMA1, SRN9, CD1, CTR, UNT2 ecotypes.
| Ecotypes | Fe | Zn | Mn | Cu | Mg | K | Na | Ca |
|---|---|---|---|---|---|---|---|---|
| NMA1 | 71.17 ± 2.69 a | 26.33 ± 0.95 a | 8.18 ± 0.30 a | 12.27 ± 0.52 a | 42.54 ± 0.93 a | 846.47 ± 19.85 a | 4.09 ± 0.13 a | 28.63 ± 0.86 a |
| CD1 | 70.07 ± 2.65 a | 67.05 ± 1.56 b | 34.35 ± 1.16 b | 41.22 ± 0.73 b | 164.88 ± 6.55 b | 2382.24 ± 29.95 b | 6.87 ± 0.13 b | 70.07 ± 1.62 b |
| CTR | 40.70 ± 1.82 b | 17.83 ± 0.84 c | 7.85 ± 0.15 ac | 11.42 ± 0.42 ac | 38.56 ± 0.69 ac | 638.10 ± 6.28 c | 3.57 ± 0.09 c | 17.85 ± 0.46 c |
| SRN9 | 58.09 ± 1.15 c | 45.93 ± 2.19 d | 9.46 ± 0.08 d | 14.86 ± 0.65 d | 91.87 ± 1.54 d | 2004.88 ± 33.34 d | 6.76 ± 0.18 b | 58.09 ± 1.05 d |
| UNT2 | 52.02 ± 1.17 d | 18.85 ± 0.90 c | 8.45 ± 0.21 acd | 10.40 ± 0.30 c | 41.60 ± 0.84 ac | 570.83 ± 12.13 e | 3.25 ± 0.04 c | 41.60 ± 0.72 e |
Each value represents the means ± SEM of three replicates, n = 3, and different letters on the same column indicate significant difference using Tukey test at 0.05 level of significance (p < 0.05).
3. Metabolite Profiling using UHPLC-PDA-ESI-OT-MS
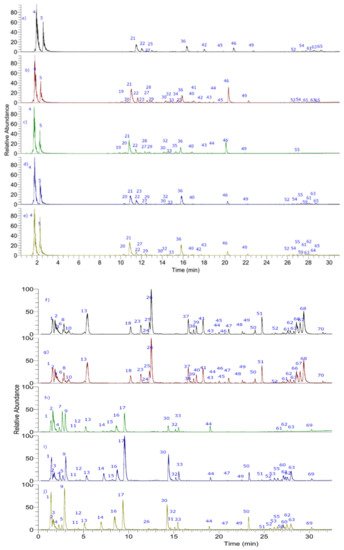
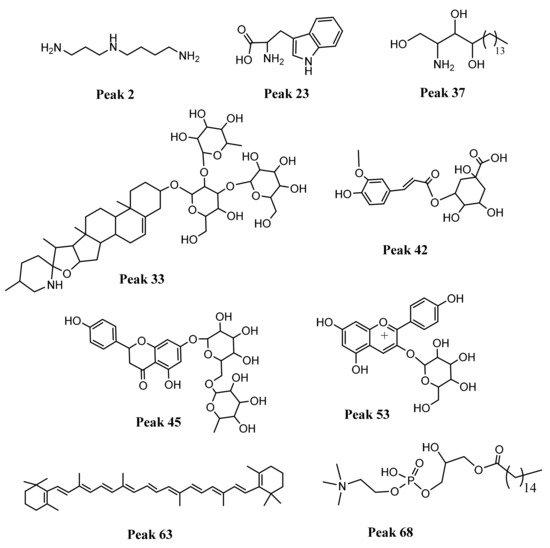
The metabolite profiling was comprehensively performed by UHPLC-PDA-ESI-OT-MS, while Figure 2 shows the base peak UHPLC-mass chromatograms of ecotypes of cocona fruits and Table 3 shows the tentative identification of metabolites detected in the five ecotypes. Below are the detailed analyses, while Figure 3 show the structures of some representative compounds.

Figure 2. UHPLC-PDA-ESI-OT-MS chromatograms (TIC, total ion current) of cocona fruits NMA1, SRN9, CD1, CTR, UNT2 ecotypes: (a–e) positive mode and (f–j) negative mode.

Figure 3. Structures of some representative compounds detected in cocona ecotypes: spermidine, peak 2, the aminoacid triptophan, peak 23, phytoesphingosine peak 37, spirosol-5-en-3-ol, 3-O-[rhamnosyl-glucosyl]-galactoside, peak 33, 3-O-feruloylquinic acid, peak 42, naringenin 7-O-rutinoside, peak 45, pelargonidin 3-O-glucoside, peak 53, B-carotene, peak 63 and 1-hexadecanoyl-sn-glycero-3-phosphocholine, peak 68.
Table 3. High resolution UHPLC-PDA-ESI-OT-MS identification of metabolites in fractions of cocona fruits (a–e): NMA1, SRN9, CD1, CTR, UNT2 ecotypes, respectively.
| Peak# | Retention Time (min.) | UV Max | Tentative Identification |
Molecular Formula | Theoretical Mass (m/z) | Measured Mass [M-H]− or [M+H]+ (m/z) | Accuracy (ppm) |
MSn Ions | Ecotype |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1.25 | - | Spermine | C10H26N4 | 203.22302 | 203.2229 | −2.448 | 129.1385, 112.1122, 84.0812, 73.0813 | a–e |
| 2 | 1.33 | - | Spermidine | C7H19N3 | 146.16517 | 146.1651 | −1.96 | 129.1385, 112.1122, 84.0812, 73.0813 | a–e |
| 3 | 1.35 | - | Histamine | C5H9N3 | 112.08692 | 112.0872 | 0.79 | 95.0606, 83.0608, 68.0500, 55.0549 | a–e |
| 4 | 1.69 | - | Citric acid | C6H8O7 | 191.01944 | 191.01863 | 4.24 | 129.1385 | c–e |
| 5 | 1.75 | - | Isocitric acid | C6H8O7 | 191.01944 | 191.01947 | 3.25 | 111.00794 | d–e |
| 6 | 1.97 | - | Asparagine | C4H8N2O3 | 131.0449 | 131.0454 | 3.81 | 114.0187, 113.0337, 95.0251, 88.0394 70.0288 |
a–b |
| 7 | 2.29 | - | Arginine | C6H14N4O2 | 175.1181 | 175.1188 | 3.99 | 158.0920, 140.0702, 130.0972, 116.0706 97.0661 70.0656 |
c |
| 8 | 2.43 | - | Pyroglutamic acid | C5H7NO3 | 130.0493 | 130.0499 | 4.61 | 102.0251, 84.0448, 56.0551, | a–b |
| 9 | 3.01 | - | Nicotinamide | C6H6N2O | 123.0550 | 123.0553 | 2.43 | 106.0289, 96.0446, 80.0499, 53.0391 | c–e |
| 10 | 3.15 | - | N-phenyl ethyl amide | C8H10N | 120.08810 | 120.08080 | 2.42 | 85.02876 | a–b |
| 11 | 4.1 | - | N-Fructosyl isoleucine | C12H23NO7 | 294.1541 | 294.1544 | 1.01 | 276.1436, 258.1332, 230.1383, 212.1278 161.0681, 144.1017, 132.1017, 86.0968 |
c–e |
| 12 | 4.32 | - | Norleucine | C6H13NO2 | 132.1016 | 132.1019 | 2.27 | 105.0696, 86.0968, 69.0704 | c–e |
| 13 | 5.21 | - | Tyrosine | C9H11NO3 | 188.0210 | 188.0211 | 0.53 | 165.0554, 147.0438, 136.0755, 123.0441 105.0337 |
a–e |
| 14 | 7.03 | - | Adenosine | C10H13N5O4 | 268.1037 | 268.1039 | 0.74 | 178.0730, 136.0616, 57.0341 | c–e |
| 15 | 8.12 | - | Phenylacetaldehyde | C8H8O | 121.0642 | 121.0649 | 5.48 | 103.0544, 93.0702, 91.0546, 77.0387 53.0392 |
c–e |
| 16 | 8.53 | - | Guanosine | C10H13N5O5 | 284.0983 | 284.0988 | 1.75 | 152.0564, 133.0494, 121.0647, 95.0609 | c–e |
| 17 | 9.62 | - | Phenylalanine | C9H11NO2 | 166.0859 | 166.0861 | 1.20 | 149.0594, 131.0491, 120.0808, 103.0543 93.0703 |
c–e |
| 18 | 10.23 | - | Aminobutyl benzamide | C11H16N2O | 193.1332 | 193.1336 | 2.07 | 176.1067, 134.0599, 105.0337, 72.0813 | a–b |
| 19 | 10.34 | 290–335 | Chlorogenic acid | C8H14O4− | 353.0863 | 353.0882 | 4.21 | 191.05574, 707.18678 | b–c |
| 20 | 10.5 | 208 | Quinic acid | C7H11O6− | 191.0550 | 191.0557 | 2.34 | 135.04477, 85.02844 | b–e |
| 21 | 10.88 | 235 | 3-O-diglucosyl-4-methoxy-3-hydroxybenzoic acid | C20H27O14− | 491.1395 | 491.1412 | 3.46 | a–d | |
| 22 | 11.25 | - | Pantothenic acid | C9H17NO5 | 220.1173 | 220.1179 | 2.72 | 202.1069, 184.0965, 160.0965, 142.0860 124.0756 |
a–d |
| 23 | 11.43 | - | Tryptophan | C11H12N2O2 | 205.0961 | 205.0968 | 3.41 | 188.0701, 170.0596, 159.0914, 146.0597 132.0804 118.0650 |
a–b |
| 24 | 11.75 | - | Tryptophol | C10H11NO | 144.0802 [M- H2O+H] |
144.0807 | 3.47 | 128.0491, 117.0699, 103.0506, 91.0547 | a–b |
| 25 | 11.97 | 330 | N-Caffeoyl-N-(dihydrocaffeoyl)spermidine | C25H33N3O6 | 472.2439 | 472.2441 | 0.42 | 455.2163, 310.2118, 293.1852, 222.1120 163.0386, 72.0813 |
a |
| 26 | 12.03 | 330 | N-Caffeoyl-N-(dihydrocaffeoyl)spermidine | C26H37N3O6 | 488.2751 | 488.2756 | 1.02 | 471.2478, 324.2273, 293.1844, 236.1275 222.1119, 165.0542 |
a–b |
| 27 | 12.02 | 325 | 3-O-Diglucosyl-4-methoxy-3-hydroxybenzoic acid | C20H27O14− | 491.13953 | 491.14124 | 3.46 | a–d | |
| 28 | 12.24 | 254–354 | Rutin | C27H30O16 | 609.14702 | 609.14709 | 0.11 | 463.0920, 343.0465, 301.0254, 300.0280, 271.0252 178.9982, 151.0031 |
b–c |
| 29 | 12.46 | 240 | Apiosyl-(1→6)-glucosyl 4-hydroxybenzoate | C18H24O12 | 431.0980 | 431.0983 | 0.46 | 431.1196, 299.0768, 281.0679, 137.0237 93.0336 |
b–d |
| 30 | 14.03 | 280 | Naringenin-5,7-di-O-D-glucopyranoside | C27H31O15− | 595.16575 | 595.16772 | 3.32 | 271.06152, 153.01845, 147.04482, 119.05661 | c–e |
| 31 | 14.21 | 280 | Genistein 5-O-glucoside | C21H20O10 | 431.0980 | 431.0984 433.1123 |
0.46 | 414.3355, 271.0595, 269.0390, 253.0485, 215.0698 146.0598, 127.0389, 85.0288 |
d–e |
| 32 | 15.02 | 254–354 | Isoquercitrin | C21H20O12 | 463.1022 | 463.1027 | 1.08 | 300.0280, 271.0251, 255.0301, 178.9982 151.0032 |
c–e |
| 33 | 15.21 | - | Spirosol-5-en-3-ol, 3-O-[Rhamnosyl-(1→2)- glucosyl-(1→3)]-galactoside |
C45H73NO16 | 884.4982 | 884.4987 928.4909 [M+FA-H] |
0.56 | 722.4060, 576.3906, 414.3356 | b–e |
| 34 | 15.24 | 329 | 1-O-Sinapoyl-glucoside | C17H22O10 | 385.1142 | 385.1147 | 1.29 | 247.0612, 223.0611, 205.0504, 190.0269 164.0704, 119.0342, |
b |
| 35 | 15.36 | 280 | Protocatechuic acid 5-O-[apiofuranosyl-(1→6)-glucopyranoside] | C19H26O13 | 461.1301 | 461.1302 | 0.21 | 329.0872, 167.0344, 152.0108, 123.0443 108.0208 |
b–c |
| 36 | 15.57 | 325 | 4-O-(3′-O-Glucopyranosyl)-caffeoyl quinic acid | C22H28O14 | 515.1401 | 515.1407 | 1.16 | 395.0990, 353.0876, 191.0557, 179.0344 161.0238, 135.0444 |
a–e |
| 37 | 15.87 | - | Phytosphingosine | C18H39NO3 | 318.2990 | 318.2995 | 1.57 | 300.2890, 282.2785, 270.2785, 60.0450 | a, b, d, e |
| 38 | 16.23 | 280 | N,N″-Bis[3-(4-hydroxy-3-methoxyphenyl)propanoyl] spermidine | C27H39N3O6 | 502.2902 | 502.2907 | 0.99 | 485.2633, 307.1996, 236.1275, 179.0698 137.0594 |
a–b |
| 39 | 16.73 | 325 | N,N,N-tris(dihydrocaffeoyl) spermidine | C34H43O9N3 | 638.3059 | 638.3062 | 0.46 | 474.2588, 456.2484, 293.1852, 222.1120 165.0543, 123.0439 |
a–b |
| 40 | 17.23 | - | Spirosol-5-en-3-ol, O-[Rhamnosyl-(1→2)-[xylosyl- (1→2)-rhamnosyl-(1→4)]-galactoside |
C50H81NO19 | 1000.5450 | 1000.5456 | 0.59 | 868.4970, 722.4737, 576.3879, 414.3358 | a–b |
| 41 | 17.55 | - | Cholest-5-ene-3,16,22,26-tetrol, 3-O-[Rhamnosyl- (1→4)-[rhamnosyl-(1→2)]glucoside], 26-O- glucoside | C51H86O22 | 1051.5660 | 1051.5665 | 0.47 | 1049.5541, 903.4961, 757.4382, 595.3851 433.3324 |
a–b |
| 42 | 17.67 | 330 | 3-O-Feruloylquinic acid | C17H20O9 | 367.1032 | 367.1039 | 1.90 | 191.0558, 173.0451, 134.0366, 111.0443 93.0336, |
a–b |
| 43 | 18.05 | 329 | 2-O-Sinapoyl-glucoside | C17H22O10 | 385.1141 | 385.1147 | 1.55 | 247.0612, 223.0611, 205.0504, 190.0269 164.0704, 119.0342 |
a–b |
| 44 | 19.01 | 330 | Syringaresinol 4-gentiobioside | C34H46O18 | 787.2676 | 787.2678 [M+FA-H] | 0.25 | 417.1560, 402.1323, 387.1069, 371.1494 356.1233, 181.0502, 166.0266 |
a–e |
| 45 | 19.53 | 280 | Naringenin 7- O-rutinoside | C27H31O14− | 579.17083 | 579.17291 | 3.59 | 271.06152, 151.00319 | a–b |
| 46 | 19.72 | 254–354 | Quercetin 3-galactoside | C12H19O5− | 243.12270 | 463.08893 | 3.94 | 350.20898, 301.02795, 151.00310 | a–e |
| 47 | 20.45 | - | Spirosol-5-en-3-ol, 3-O-[Rhamnosyl-(1→2)-[rhamnosyl-(1→4)]-glucoside] | C45H73NO15 | 868.5031 | 868.5035 | 0.46 | 722.4411, 576.3845, 414.3357 | a, b, d, e |
| 48 | 22.32 | 280 | Biochanin A 7-O-rutinoside | C28H32O14 | 593.1852 | 593.1859 | 1.18 | 447.1269, 327.0856, 285.0750, 153.0191 | a, b |
| 49 | 22.51 | 280 | Genistin | C21H20O10 | 431.0982 | 433.1123 431.0984 |
0.69 | 414.3355, 271.0595, 253.0485, 215.0698 146.0598, 127.0389, 85.0288, |
a–e |
| 50 | 23.35 | 255–340 | 3,5-Dihydroxy-4′,7-dimethoxyflavone 3-O-[Rhamnosyl-(1→2)-glucoside (Pectolarin) | C29H34O15 | 623.1961 | 623.1963 | 0.32 | 477.1342, 315.0815, 300.0622, 284.0679 | a, b, d, e |
| 51 | 24.93 | 281 | Naringenin-7-O-glucoside | C21H22O10 | 435.1279 | 435.1282 433.1117 |
0.95 | 313.0506, 271.0617, 193.0138, 151.0032 119.0405 |
d–e |
| 52 | 25.5 | 520 | Pelargonidin 3-O-sophoroside | C27H31O15 | 595.1652 | 595.1656 | 0.67 | 433.1080, 271.0595, 215.0695, 163.0596 127.0389 |
a–b |
| 53 | 26.1 | 520 | Pelargonidin 3-O-glucoside | C21H20O10 | 433.1121 | 433.1126 | 1.15 | 311.0556, 269.0460, 163.0031 | a–b |
| 54 | 26.5 | 325 | Methyl chlorogenate | C17H20O9 | 367.1031 | 367.1035 | 1.08 | 191.0556, 179.0344, 161.0237, 135.0443 93.0335 |
a–d |
| 55 | 26.7 | - | Peak 55, 1-Hexadecanoyl-sn-glycero-3-Phosphoethanolamine | C21H44NO7P | 454.2920 | 454.2923 452.2786 |
0.66 | 255.2630, 214.0482, 214.0284, 140.0111 | c–e |
| 56 | 27.0 | 280 | Naringenin-5-O-glucoside | C21H22O10 | 433.1132 | 433.1138 | 1.38 | 313.0551, 271.0613, 151.0030, 119.0493 93.00335 |
a–e |
| 57 | 27.1 | 280 | Eriodictyol-7-O-glucoside | C21H22O10 | 449.1080 | 449.1083 | 0.66 | 287.0567, 205.0144, 175.0033, 151.0032 135.0445, 125.0237 |
a–e |
| 58 | 27.2 | - | 1-Hexadecanoyl-sn-glycero-3-phospho-(1′-myo-inositol) | C25H49O12P | 573.3030 | 573.3034 571.2089 |
0.69 | 391.2256, 333.0594, 315.0487, 255.2329 241.0118, 152.9951 |
a–e |
| 59 | 27.3 | 330 | Syringaresinol-glucoside | C28H36O13 | 579.2080 | 579.2084 | 0.89 | 417.1557, 402.1320, 387.1085, 223.0616 181.0501, 166.0265 |
c |
| 60 | 27.5 | - | 1-(9Z-Octadecenoyl)-sn-glycero-3-phospho-(1′-myo-inositol) | C27H51O12P | 599.3189 | 599.3192 597.3046 |
0.50 | 333.0594, 315.0488, 281.2487, 259.0223 241.0118 |
d–e |
| 61 | 27.7 | 254–354 | Quercetin 3-O-malonylglucoside | C24H22O15 | 579.2080 | 579.2084 | 0.69 | 463.0888, 300.0280, 271.0252, 255.0301 178.9981, 151.0032 |
a–e |
| 62 | 27.9 | 450 | Lutein | C40H56O2 | 568.4280 | 568.4282 | 124.08689, 145.0845, 105.08564, 335.12485 | a–e | |
| 63 | 28.0 | 450 | β-carotene | C40H56 | 536.4379 | 536.4382 | 337.09189, 476.17609 | a–e | |
| 64 | 28.1 | 280 | Naringenin | C15H12O5 | 271.06010 | 271.06155 | 5.36 | 153.01832, 147.04453, 119.05632 | e |
| 65 | 28.3 | 280 | Phloretin | C15H14O5 | 349.18569 | 349.18723 | 4.03 | 229.0871, 179.0347, 167.0347, 125.0237 | e |
| 66 | 28.4 | 254–354 | Cirsimarin | C23H24O11 | 475.12668 | 475.12524 | 4.36 | 315.07642 | c–e |
| 67 | 28.9 | - | 1-(9Z-Octadecenoyl)-sn-glycero-3-phosphoethanolamine | C23H46NO7P | 480.3080 | 480.3085 478.2937 |
0.83 | 281.2486, 214.0482, 196.0386, 152.9950 140.0110 |
a–b |
| 68 | 29.2 | - | 1-hexadecanoyl-sn-glycero-3-phosphocholine | C24H50NO7P | 496.3391 | 496.3396 | 1.00 | 313.2128, 184.0721, 124.9998, 104.1072 86.0968 |
a–b |
| 69 | 29.7 | 215 | 1-(9Z-Octadecenoyl)-sn-glycero-3-phosphocholine | C26H52NO7P | 566.3419 | 566.3422 [M+FA-H] 522.3555 |
0.56 | 504.3450, 445.2715, 339.2892, 240.1001 199.0370, 187.0732, 124.9988 |
c–e |
| 70 | 30.2 | 210 | 1-Oleoyl-2-palmitoyl-sn-glycero-3-phosphocholine | C42H82NO8P | 760.5832 | 760.5839 758.542 |
0.92 | 184.0730, 125.9997, 104.1071, 86.0964 | a–b |
4. Antioxidant Activity, Total Carotenes and Total Polyphenols Content
In this study the antioxidant capacities of the five ecotypes were assessed by the trapping of ABTS and DPPH and expressed as µmol Trolox/g dry matter (Table 4). In addition, total phenolic contents by the Folin and Ciocalteau’s method plus the total carotenes was assessed and correlated with the antioxidant capacities. Trapping of ABTS and DPPH radicals showed similar values in the different ecotypes and correlated with carotene and phenolic contents for the dry pulps. Strong correlation was found between total phenolics and DPPH antioxidant assays (r = 0.847, p < 0.0001). Moderated correlation was found between total carotenoids and ABTS antioxidant assays used (r = 0.640, p < 0.011). Ecotype UNT2 showed the highest DPPH• radical scavenging capacity in terms of content of Trolox equivalents (23.29 ± 1.07 µmol Trolox/g), but interestingly, in ABTS• radical scavenging activity the most powerful was the CD1 ecotype (25.67 ± 0.28 µmol Trolox/g).
Table 4. Antioxidant activity (DPPH, ABTS), total phenolic content and total carotenoid contents of cocona fruits pulps NMA1, SRN9, CD1, CTR, UNT2 ecotypes.
| Ecotypes | DPPH (µmol Trolox/g) |
ABTS (µmol Trolox/g) |
Total Phenolics (mg GAE/g) |
Total Carotenoids (μg β-carotene)/g) |
|---|---|---|---|---|
| NMA1 | 19.88 ± 0.34 a | 19.70 ± 0.81 a | 32.68 ± 1.33 a | 101.22 ± 4.47 a |
| CD1 | 18.37 ± 0.24 b | 25.67 ± 0.28 b | 28.03 ± 0.90 b | 122.65 ± 4.24 b |
| CTR | 21.92 ± 0.53 c | 21.98 ± 0.90 ac | 35.79 ± 0.84 ac | 85.13 ± 1.81 c |
| SRN9 | 18.21 ± 0.24 bd | 23.97 ± 1.12 bc | 27.86 ± 0.81 b | 92.12 ± 3.64 ac |
| UNT2 | 19.15 ± 0.84 abd | 20.25 ± 0.79 ac | 34.26 ± 1.32 ac | 58.81 ± 0.46 ab |
Each value represents the means ± SEM of three replicates, n = 3, while different letters on the same column indicate significant difference using Tukey test at 0.05 level of significance (p < 0.05).
5. Antihyperlipidemic Activities
Table 5 shows the variations in cholesterol, triglycerides, HDL and LDL in the different study groups. The highest cholesterol and triglyceride values correspond to the hypercholesteremic group that only received saline treatment (group II), with a statistically significant difference (p < 0.05) with the group control (group I) for the variables considered, thus corroborating the effectiveness of the model used. The increases obtained with Triton were 370 and 600 % for cholesterol and triglycerides, respectively. Several authors report similar elevations in the lipid profile of rodents treated with this agent. For instance, Harnafi et al. [6], obtained elevations of 270% for cholesterol and up to more than 1000% for triglycerides; while Khanna et al. [7], reported an elevation of cholesterol by 134%, these differences seem to depend on the strain of animals used.
Table 5. Effect of cocona fruit pulps NMA1, CD1, CTR, SRN9, UNT2 in serum biochemical parameters on Triton induced hyperlipidemic rat.
| Groups | Cholesterol | Triglyceride | HDL | LDL |
|---|---|---|---|---|
| Group I (control) | 78.9 ± 5.90 | 72.28 ± 3.98 | 50.97 ± 4.02 | 27.97 ± 5.26 |
| Group II hypercholesterolemic, saline treated | 378.17 ± 6.27 a | 461.65 ± 8.82 a | 42.68 ± 3.14 | 225.64 ± 12.16 a |
| Group III hypercholesterolemic, atorvastatin treated | 118.41 ± 10.05 b | 93.90 ± 11.23 b | 60.13 ± 5.08 b | 50.79 ± 4.46 b |
| Group IV hypercholesterolemic, NMA1 pulp treated | 302.76 ± 17.87 a | 268.90 ± 7.92 a | 47.44 ± 5.06 | 145.33 ± 9.56 a |
| Group V hypercholesterolemic, CD1 pulp treated | 287.28 ± 10.03 a | 259.63 ± 13.24 a | 48.12 ± 8.07 | 139.06 ± 8.07 a |
| Group VI hypercholesterolemic, CTR pulp treated | 130.09 ± 8.55 b | 108.51 ± 10.04 b | 57.30 ± 5.72 b | 65.41 ± 7.68 b |
| Group VII hypercholesterolemic, SRN9 pulp treated | 126.74 ± 6.63 b | 102.11 ± 9.47 b | 58.16 ± 6.64 b | 61.05 ± 4.00 b |
| Group VIII hypercholesterolemic, UNT2 pulp treated | 338.81 ± 15.95 ac | 299.86 ± 17.81 ac | 45.56 ± 7.46 c | 165.85 ± 7.42 ac |
Values represent the mean ± SD for observations made on six rats in each group. Units: milligrams per deciliter. a Statistically significant difference (p < 0.05) when compared with group I values, b Statistically significant difference (p < 0.05) when compared with group II values, c Statistically significant difference (p < 0.05) when compared with group III values.
When comparing the groups treated with the pulps, we noticed that samples which has received the CTR and SRN9 pulps showed the lowest cholesterol and triglyceride levels after treatment, however there is no clear correlations with the compounds detected and bioactivity showed by pulps. In agreement to the above, there isn’t a statistically significant difference (p < 0.05) between the cholesterol and triglyceride levels of the hypercholesterolemic group that received treatment with atorvastatin and the groups that received treatment with CTR and SRN9 pulps; which suggests that the treatment of the experimental animals with two pulps brings cholesterol levels closer to their basal values; this result is very important since it is an indicator of the effectiveness of the pulps tested. Regarding carotene compounds present in cocona fruits, it has been proved that diet with carotenes at a dose 80 mg/kg in rats decreased the lipid serum concentrations and its effect was comparable to that of simvastatin [8]. Regarding anthocyanins, also detected in cocona fruits, a juice from the fruit Aronia containing 106.8 mg cyanidin-3-glucoside equivalents/100 mL juice and 709.3 mg gallic acid equivalents/100 mL juice, showed an antihyperlipidemic effect in rats with hyperlipidemia and was proved to be valuable in reducing factors of cardiovascular risks [9]. Moreover, mulberry (Morus alba L.) lyophilized fruit administered to rats proved to have significant decrease in levels of serum and liver triglycerides, total cholesterol, serum low-density lipoprotein cholesterol, and a decrease in the atherogenic index, while the serum high-density lipoprotein cholesterol was significantly increased [10]. Several flavonoids and extracts rich in flavonoids were attributed anti hyperlipidemia activities; Pterocarpus marsupium heartwood and its flavonoid constituents, marsupsin, pterosupin, and liquiritigenin were able to effect a significant fall in serum cholesterol, LDL-cholesterol, and atherogenic index[11], also extracts full of glycosylated flavonoids from Cardoncellus marioticus showed antioxidant and antihyperlipidemic activities [12] moreover, using our same triton induced hyperlipidemia system extracts from the Mediterranean buckthorn, Rhamnus alaternus full of flavonoids decreased blood levels of cholesterol and triacylglycerols in hyperlipidemic rats (by 60% and 70%, respectively, at 200 mg CME/kg) [13]. In this study, all groups treated with the pulps showed a significant reduction in LDL-C (p < 0.05) with respect to the negative control group (group II), with the SRN9 group showing the best results. Despite the reduction in total cholesterol obtained with the different pulp treatments, we can observe that none of the treatments achieved lower values than the group treated with atorvastatin. In all the groups evaluated, an increase in HDL-C concentration was observed, the increase being significant in the groups that received CTR and SRN9 pulp (p < 0.05); the best result obtained was in the group that received SRN9 pulp after 96 hours of treatment. From the above mentioned we can say that SRN9 pulp, presents the best result of inhibition of the surfactant used (Triton WR-1339), by significantly decreasing the serum concentrations of cholesterol and triglycerides, corroborating the traditional use of this species and previous reports on its antihyperlipidemic activity [14][15]. This allows us to affirm that SRN9 pulp at the doses tested shows an antihyperlipidemic effect and could be an option in the management of people with high lipid levels. The presence of phenolic compounds, including flavonoids, could explain the hypolipidemic effect shown, presumably due to their proven antioxidant activity, as a result of a combination of their iron chelating and free radical scavenging properties [16][17]. During the experimental phase, no toxic effects were evidenced in the experimental specimens treated with NMA1; CD1; CTR; SRN9 and UNT2 pulps.
This entry is adapted from the peer-reviewed paper 10.3390/antiox10101566
References
- Rodrigues, E.; Mariutti, L.R.B.; Mercadante, A.Z. Carotenoids and phenolic compounds from Solanum sessiliflorum, an unexploited amazonian fruit, and their scavenging capacities against reactive oxygen and nitrogen species. J. Agric. Food Chem. 2013, 61, 3022–3029.
- Mascato, D.R.D.L.H.; Monteiro, J.B.; Passarinho, M.M.; Galeno, D.M.L.; Cruz, R.J.; Ortiz, C.; Morales, L.; Lima, E.S.; Carvalho, R.P. Evaluation of Antioxidant Capacity of Solanum sessiliflorum (Cubiu) Extract: An in Vitro Assay. J. Nutr. Metab. 2015, 2015, 1–8.
- Vargas-Muñoz, D.P.; Cardoso da Silva, L.; Neves de Oliveira, L.A.; Teixeira Godoy, H.; Kurozawa, L.E. 5-caffeoylquinic acid retention in spray drying of cocona, an Amazonian fruit, using hydrolyzed collagen and maltodextrin as encapsulating agents. Dry. Technol. 2020, 39, 1854–1868.
- Faria, J.V.; Valido, I.H.; Paz, W.H.P.; da Silva, F.M.A.; de Souza, A.D.L.; Acho, L.R.D.; Lima, E.S.; Boleti, A.P.A.; Marinho, J.V.N.; Salvador, M.J.; et al. Comparative evaluation of chemical composition and biological activities of tropical fruits consumed in Manaus, central Amazonia, Brazil. Food Res. Int. 2021, 139, 109836.
- Dos Santos Montagner, G.F.F.; Barbisan, F.; Ledur, P.C.; Bolignon, A.; De Rosso Motta, J.; Ribeiro, E.E.; De Souza Praia, R.; Azzolin, V.F.; Cadoná, F.C.; Machado, A.K.; et al. In Vitro Biological Properties of Solanum sessiliflorum (Dunal), an Amazonian Fruit. J. Med. Food 2020, 23, 978–987.
- Harnafi, H.; Bouanani, N.e.H.; Aziz, M.; Serghini Caid, H.; Ghalim, N.; Amrani, S. The hypolipidaemic activity of aqueous Erica multiflora flowers extract in Triton WR-1339 induced hyperlipidaemic rats: A comparison with fenofibrate. J. Ethnopharmacol. 2007, 109, 156–160.
- Khanna, A.K.; Rizvi, F.; Chander, R. Lipid lowering activity of Phyllanthus niruri in hyperlipemic rats. J. Ethnopharmacol. 2002, 82, 19–22.
- Sundaram, R.; Ayyakkannu, P.; Muthu, K.; parveen Nazar, S.; Palanivelu, S.; Panchanatham, S. Acyclic Isoprenoid Attenuates Lipid Anomalies and Inflammatory Changes in Hypercholesterolemic Rats. Indian J. Clin. Biochem. 2019, 34, 395–406.
- Valcheva-Kuzmanova, S.; Kuzmanov, K.; Mihova, V.; Krasnaliev, I.; Borisova, P.; Belcheva, A. Antihyperlipidemic effect of Aronia melanocarpa fruit juice in rats fed a high-cholesterol diet. Plant Foods Hum. Nutr. 2007, 62, 19–24.
- Yang, X.; Yang, L.; Zheng, H. Hypolipidemic and antioxidant effects of mulberry (Morus alba L.) fruit in hyperlipidaemia rats. Food Chem. Toxicol. 2010, 48, 2374–2379.
- Jahromi, M.A.F.; Ray, A.B.; Chansouria, J.P.N. Antihyperlipidemic effect of flavonoids from pterocarpus marsupium. J. Nat. Prod. 1993, 56, 989–994.
- Shabana, M.M.; El-Sherei, M.M.; Moussa, M.Y.; Sleem, A.A.; Abdallah, H.M. Flavonoid constituents of Carduncellus mareoticus (Del.) Hanelt and their biological activities. Nat. Prod. Commun. 2008, 3, 779–784.
- Tacherfiout, M.; Petrov, P.D.; Mattonai, M.; Ribechini, E.; Ribot, J.; Bonet, M.L.; Khettal, B. Antihyperlipidemic effect of a Rhamnus alaternus leaf extract in Triton-induced hyperlipidemic rats and human HepG2 cells. Biomed. Pharmacother. 2018, 101, 501–509.
- Maia, J.R.P.; Schwertz, M.C.; Sousa, R.F.S.; Aguiar, J.P.L.; Lima, E.S. Efeito hipolipemiante da suplementação dietética com a farinha do cubiu (solanum sessiliforum dunal) em ratos hipercolesterolêmicos. Rev. Bras. Plantas Med. 2015, 17, 112–119.
- Pardo, M.A. Efecto de Solanum sessiliflorum dunal sobre el metabolismo lipídico y de la glucosa. Cienc. Investig. 2004, 7, 43–48.
- Zeashan, H.; Amresh, G.; Singh, S.; Rao, C.V. Hepatoprotective activity of Amaranthus spinosus in experimental animals. Food Chem. Toxicol. 2008, 46, 3417–3421.
- Nicholson, S.K.; Tucker, G.A.; Brameld, J.M. Effects of dietary polyphenols on gene expression in human vascular endothelial cells. Proc. Nutr. Soc. 2008, 67, 42–47.
This entry is offline, you can click here to edit this entry!
