Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Allergy
l-Arginine is a semi-essential amino acid involved in numerous biological processes. It is a substrate for different enzymatic reactions and is metabolized using three major known pathways in the body: (1) Arginase metabolizes l-Arginine to l-ornithine, (2) l-Arginine decarboxylase metabolizes l-Arginine to agmatine, and (3) nitric oxide (NO) synthase (NOS) uses l-Arginine to form NO and citrulline.
- l-Arginine
- acute respiratory syndrome
- COVID-19
- nitric oxide
1. Functional Role of l-Arginine in NO Formation
l-Arginine is the substrate used for NO production by NOS [5]; due to its ability to cause NO generation, which has been shown to be a major endothelial relaxation factor (able to increase vasodilation and reduce arterial blood pressure [4,6,7,8]), l-Arginine has considerable potential in becoming a tool to tackle cardiovascular issues [9]. For instance, in patients with known endothelial dysfunction, l-Arginine supplementation (6–8 g per day) has been shown to improve endothelial function and ultimately lower blood pressure [9].
Three isoforms of NOS have been identified; two of them (endothelial NOS [10,11] and neuronal NOS [12,13]) are expressed constitutively, while the last one is inducible and is mainly involved in the inflammatory/immune response [14,15,16,17].
In the reaction carried out by NOS, electrons are transferred to heme in the N-terminal domain [18,19]. Electrons are taken from nicotinamide adenine dinucleotide phosphate (NADP) using flavin adenine dinucleotide in the C-terminal reductase domain [20]. Once electrons are transferred to the N terminal oxygenase domain, NO and citrulline are formed via l-Arginine oxidation [5,21,22]. For NOS to function properly, there needs to be an ample amount of l-Arginine available for this reaction [23]. In addition, NADP, glutathione, tetrahydrobiopterin, and oxygen are needed for proper functioning [4,24].
A substrate competition occurs between NOS and arginase [25,26]. Although the affinity for l-Arginine in NOS is much higher than arginase, the speed of the reaction allows for substrate concentration. The speed of arginase rection is a thousand times faster than NOS [27]. Since these two enzymes compete for a common substrate, arginase will reduce the amount of L-Arginine available for NOS to use [28,29], ultimately decreasing the amount of NO produced.
2. Effects of l-Arginine on the Immune System
A large part of a normal immune system depends on the amount of l-Arginine available in the body. Arginase is known to represent an integral part of certain granulocyte subsets, which can be released locally or systematically once there is an immune response. In addition, there is an accumulation of immature myeloid cells that express arginase, which is released when fighting off specific illnesses. These myeloid cells that express arginase can decrease the amount of l-Arginine [58,59,60].
T cell function has been shown to depend on l-Arginine levels [61,62]. A decreased ability of lymphocytes to proliferate has been reported in critically ill septic patients and correlated to reduced availability of l-Arginine [63]. Moreover, l-Arginine administration has been found to be beneficial to maintain immune homeostasis (Figure 2), especially in terms of T cell and macrophage function [64]. In fact, l-Arginine is essential in the macrophage M1-to-M2 switch [3].
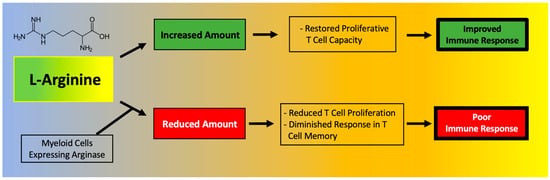
Figure 2. Main effects of l-Arginine on the immune system.
A deficiency in l-Arginine has been shown to lead to a reduction in T cell proliferation and to cause a diminished response in T cell-mediated memory [65]. In vitro assays have validated that L-Arginine can restore the function of T cells [66]. Mechanistically, the immunosuppressive effects of myeloid-derived suppressor cells (MDSCs) due to l-Arginine depletion and lymphocyte mitochondrial dysfunction have been demonstrated in models of cancer [61].
The expansion of MDSCs observed in COVID-19 has been directly correlated to enhanced arginase activity and lymphopenia [67]. Monocytic MDSCs were significantly expanded in the blood of COVID-19 patients and were strongly associated with disease severity; MDSCs were shown to suppress T cell proliferation and IFNγ production, at least in part through an arginase-dependent mechanism, strongly indicating a role for these cells in the dysregulated COVID-19 immune response [68]. Indeed, MDSCs express high levels of arginase, which metabolizes l-Arginine to ornithine and urea, effectively depleting this amino acid from the microenvironment [69]. l-Arginine depletion is known to inhibit T cell receptor signaling, eventually resulting in T cell dysfunction [70] and to increase the generation of reactive oxygen species (ROS), thereby exacerbating inflammation [69,71].
In a recent study focused on COVID-19, Dr. Claudia Morris and colleagues were able to determine the bioavailability of l-Arginine in three cohorts: asymptomatic healthy adults, adults hospitalized with COVID-19, and children hospitalized with COVID-19; they found that both adults and children affected by COVID-19 display significantly lower levels of plasma l-Arginine (as well as l-Arginine bioavailability) compared to controls [72]. Additionally, a low l-Arginine-to-ornithine ratio observed in COVID-19 patients [72] indicates an elevation of arginase activity in these patients. In another study, plasmatic L-Arginine levels were shown to inversely correlate with the severity of COVID-19 [73]. This study also revealed that the expression of the activated GPIIb/IIIa complex (PAC-1), known to be involved in platelet activation and thromboembolic events [74], is higher on platelets of patients with severe COVID-19 compared to healthy controls and inversely correlated with the plasmatic concentration of l-Arginine [73].
These pieces of evidence seem to go against the recently proposed strategy of l-Arginine depletion in COVID-19, based on the assumption that some steps in the viral lifecycle of SARS-CoV-2 could depend on l-Arginine residues (for instance, the nucleocapsid protein has a 6.9% l-Arginine content) [75].
In fact, a decrease in the bioavailability of l-Arginine has been shown to cause a diminished T cell response and function, eventually leading to an increased susceptibility to infections [76,77]. Twelve weeks of continuous l-Arginine supplementation significantly decreased the level of IL-21 [78], while NO has been shown to suppress the proliferation and function of human Th17 cells [79], which have been implied in the pathogenesis of the cytokine storm and of hyperinflammatory phenomena observed in COVID-19 patients [80,81,82,83]. Higher l-Arginine levels are associated with lower levels of CCL-20, a ligand for CCR6, a part of the chemotaxis system that is induced in response to coronavirus infections [81].
In vitro assays have demonstrated that the proliferative capacity of T cells is significantly reduced in COVID-19 patients and can be restored through l-Arginine supplementation [67]. Corroborating these findings, recent metabolomics data indicates that l-Arginine pathways are altered in COVID-19 patients [84] and an increased mRNA expression of arginase has also been found in the peripheral blood mononuclear cells (PBMCs) of COVID-19 patients [85].
Of note, circulating levels of metabolites of the l-Arginine pathway can be affected by arginase activity in red blood cells [86], which is known to be affected by oxidative stress and can contribute to endothelial dysfunction observed in COVID-19 [87]; furthermore, l-Arginine metabolism is known to be altered in hemolysis [88]. The exquisite balance between arginase and NOS activity has also been shown to influence the inflammatory responses of gut resident macrophages [3].
To actually test l-Arginine in COVID-19 patients, based on the rationale described above, we designed a randomized clinical trial to study the effects of adding l-Arginine orally (Bioarginina®, 1.66 g twice per day) to standard therapy in patients hospitalized for COVID-19. The interim results, recently published [89], revealed that patients who received l-Arginine had a significantly reduced duration of the in-hospital stay, and a diminished respiratory support, compared to patients in the placebo arm.
This entry is adapted from the peer-reviewed paper 10.3390/nu13113951
This entry is offline, you can click here to edit this entry!
