Functional amyloids can be found in bacteria, unicellular eukaryotes, fungi, plants, insects and vertebrates, playing roles as diverse as surface protection and modification, mediation of pathogen-host interactions, pigment biosynthesis, homeostasis control, hormone storage and release, signal transduction, among others. The aggregation of a polypeptide chain into amyloid fibrils and their accumulation and deposition into insoluble plaques and intracellular inclusions is the hallmark of several misfolding diseases known as amyloidoses. Alzheimer′s, Parkinson′s and Huntington’s diseases are some of the approximately 50 amyloid diseases described to date.
- misfolding diseases
- amyloidosis
- oligomers
- aggregates
- aggregation
- aggregation mechanisms
- steric zipper
- amyloid fibrils
- amyloid structure
- amyloid dyes
1. Background
| Disease | Precursor Protein | Polypeptide Length (n° of Residues) | Structural Organization of Precursor |
|---|---|---|---|
| Neurodegenerative Diseases | |||
| Alzheimer’s disease | Amyloid-β variants | 37–44 | IDP |
| Spongiform encephalopathies | Prion protein or its fragments | 208 | IDP and α-helical |
| Parkinson’s disease | α-synuclein | 140 | IDP |
| Frontotemporal dementia with Parkinsonism | Tau | 352–441 | IDP |
| Amyotrophic lateral sclerosis | Superoxide dismutase 1 | 153 | β-sheet |
| Huntington’s disease | Huntingtin with polyQ expansion | 3144 | Mostly IDP |
| Neuroferritinopathy | Ferritin | 175 or 183 | α-helical |
| Familial British dementia | ABri | 34 | IDP |
| Familial Danish dementia | ADan | 34 | IDP |
| Familial amyloid polyneuropathy | Transthyretin variants | 127 | β-sheet |
| Non-Neuropathic Systemic Amyloidosis | |||
| Amyloid light chain amyloidosis | Immunoglobulin light chains or its fragments | ~90 | β-sheet |
| Amyloid heavy chain amyloidosis | Immunoglobulin heavy chains or its fragments | ~220 | β-sheet |
| Amyloid A amyloidosis | Serum amyloid A protein fragments | 45–104 | α-helical and unknown fold |
| Familial Mediterranean fever | Serum amyloid A protein fragments | 45–104 | α-helical and unknown fold |
| Apolipoprotein A1 amyloidosis | Apo A-1 fragments | 80–93 | IDP |
| Senile systemic amyloidosis | Wild-type transthyretin | 127 | β-sheet |
| Familial amyloid cardiomyopathy | Transthyretin variants | 127 | β-sheet |
| Haemodialysis-related amyloidosis | β2-microglobulin | 99 | β-sheet |
| Lysozyme amyloidosis | Lysozyme variants | 130 | α-helical and β-sheet |
| Finnish hereditary amyloidosis | Fragments of gelsolin variants | 53 or 71 | IDP |
| Non-Neuropathic Localized Amyloidosis | |||
| Type II diabetes | Islet amyloid polypeptide | 37 | IDP |
| Injection-localized amyloidosis | Insulin | 21 and 30 | α-helical |
| Gelatinous drop-like corneal dystrophy | Lactoferrin | 691 | α-helical and β-sheet |
| Medullary carcinoma of the thyroid | Calcitonin | 32 | IDP |
| Localized cutaneous amyloidosis | Galectin 7 | 136 | β-sheet |
| Atrial amyloidosis | Atrial natriuretic factor | 28 | IDP |
| Cataracts | γ-crystallins | variable | β-sheet |
2. Protein Aggregation
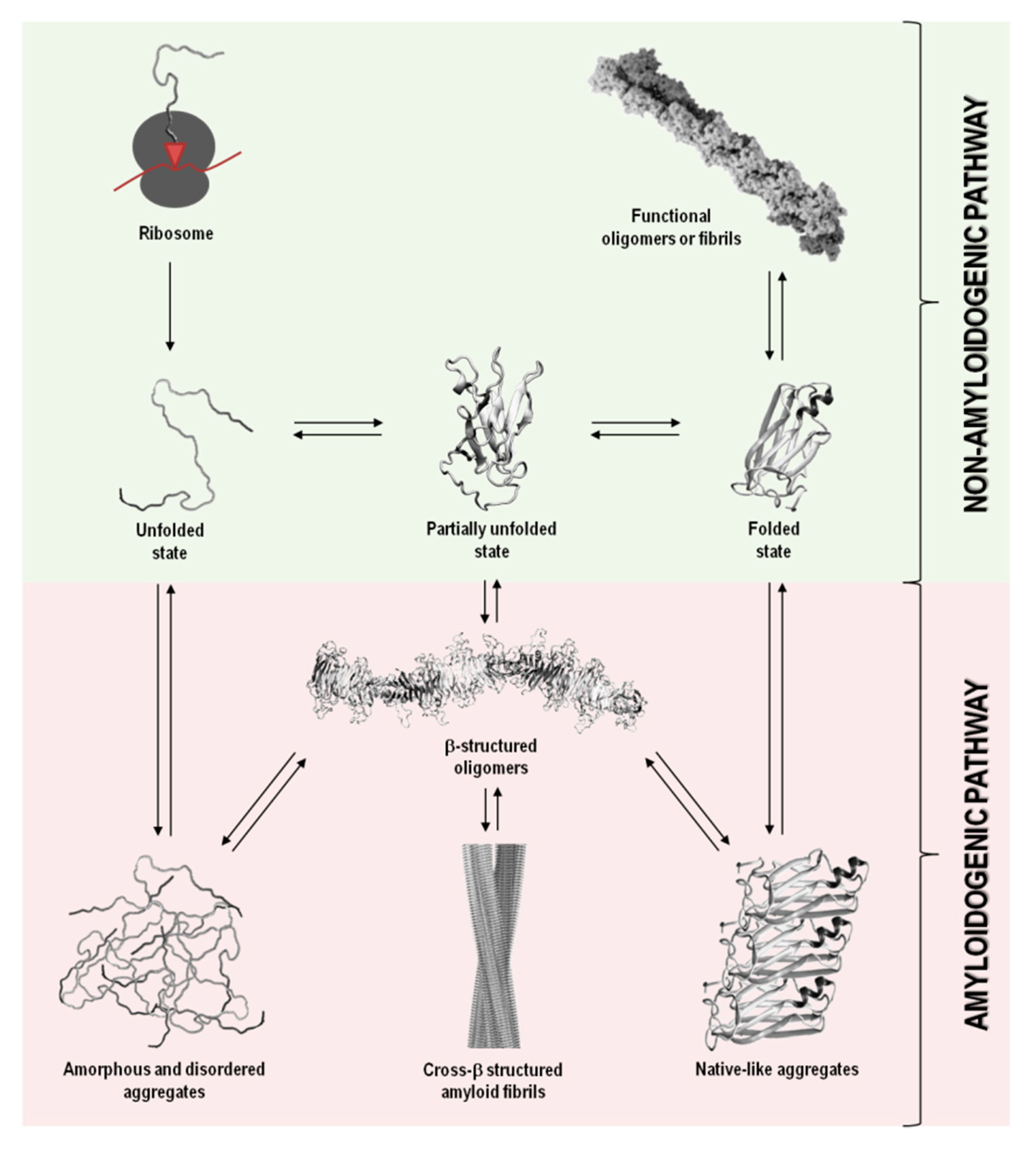
3. Amyloid Fibrils
3.1. The Tinctorial Properties of Amyloid Fibrils
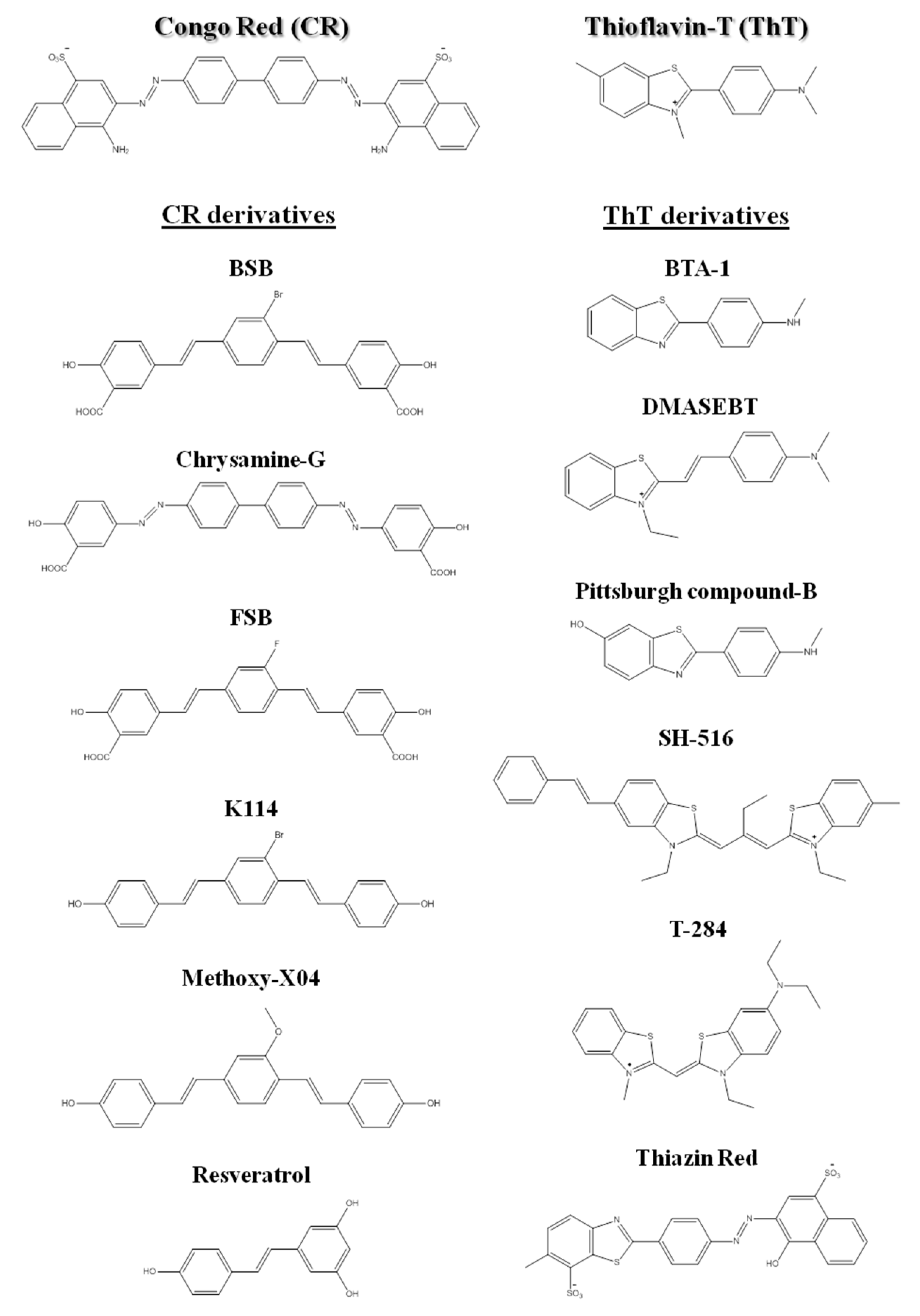
3.2. Structure of Amyloid Fibrils at the Subunit Level
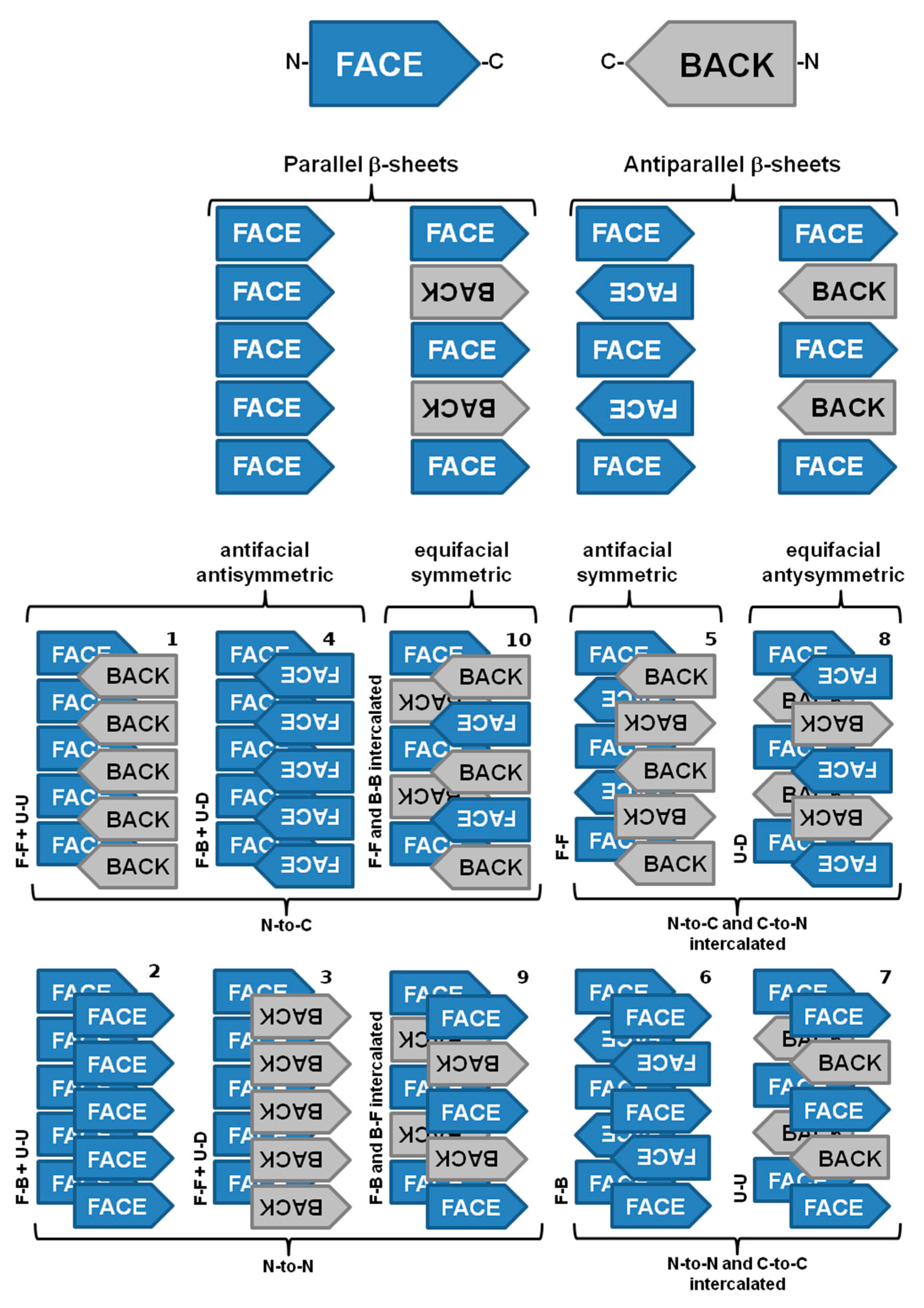
3.3. The Cross-α Amyloid-Like Fibril
3.4. Hetero-Amyloid Fibrils
3.5. The Major Differences between the Techniques that Inform on Amyloid Structure
| Technique | Advances | Advantages | Disadvantages | References |
|---|---|---|---|---|
| X-ray and electron diffraction | 1. Discovery of short protein segments that can themselves form amyloid fibrils and closely related crystals; 2. Development of synchrotron X-ray microbeams sufficiently focused and intense to determine a structure from a single crystal. |
1. May yield atomic resolution; 2. Is not limited by the molecular weight of the specimen. |
1. Well-ordered microcrystals needed; 2. The fibrils formed by some segments may represent the spines of polymorphs of full fibrils, but others may not; 3. The crystallized segment is only a few residues in length, thus nothing is revealed about the fibril structure outside the spine; 4. The steric zippers structures only show homo-steric zippers. |
[23][60][62][126] |
| ssNMR | 1. Innovations in high-field magnets, pulse sequences, high-resolution multi-channel magic-angle spinning (MAS) probes, ultrafast MAS, isotopic labeling schemes, use of quadrupolar nuclei as spectroscopic probes and solid-state dynamic nuclear polarization (DNP). | 1. No need for crystals; 2. Structural information obtained on: identity of residues, recognition of parallel versus antiparallel β-sheets, register of strands within a sheet, and inter-residue contacts of amino acid side chains; 3. ssNMR-determined models show the overall conformation of the well-ordered portion of the chain around the protofilament spine; 4. Can be used to determine dihedral angles and inter-atom distances in the fibril subunits. |
1. Amyloid-forming proteins are expressed recombinantly from media containing isotopically labeled amino acids; 2. Reliability of molecular models is highly dependent on the number of experimental constraints that have been collected; 3. The relative positions of atoms are not as accurately determined as in an atomic-resolution crystal structure; 4. The sensitivity of the experiments and spectral resolution decrease with the increase in molecular weight. |
[127][129][130] |
| cryo-EM | 1. Introduction of high-field microscopes; 2. New generation of direct detectors record the incident electrons in a thin, sensitive layer so that the signal is not scattered into surrounding pixels resulting in an improvement in image processing. |
1. Near atomic-resolution structures of large molecular complexes without the need for crystals; 2. May yield the overall fibril structure: the number of protofilaments; the degree of twist; and, depending on the number of well-ordered specimens, information on the atomic structure of the fibril. |
1. Due to a lack of contrast, images often have a very low signal-to-noise ratio, requiring highly advanced detection hardware and image processing; 2. Sample preparation can be difficult, not only to optimize thickness, but also to optimize particle distribution; 3. The most advanced cryo-EM equipment is very expensive. |
[131][132][133] |
4. Toxic Species in Amyloid Diseases
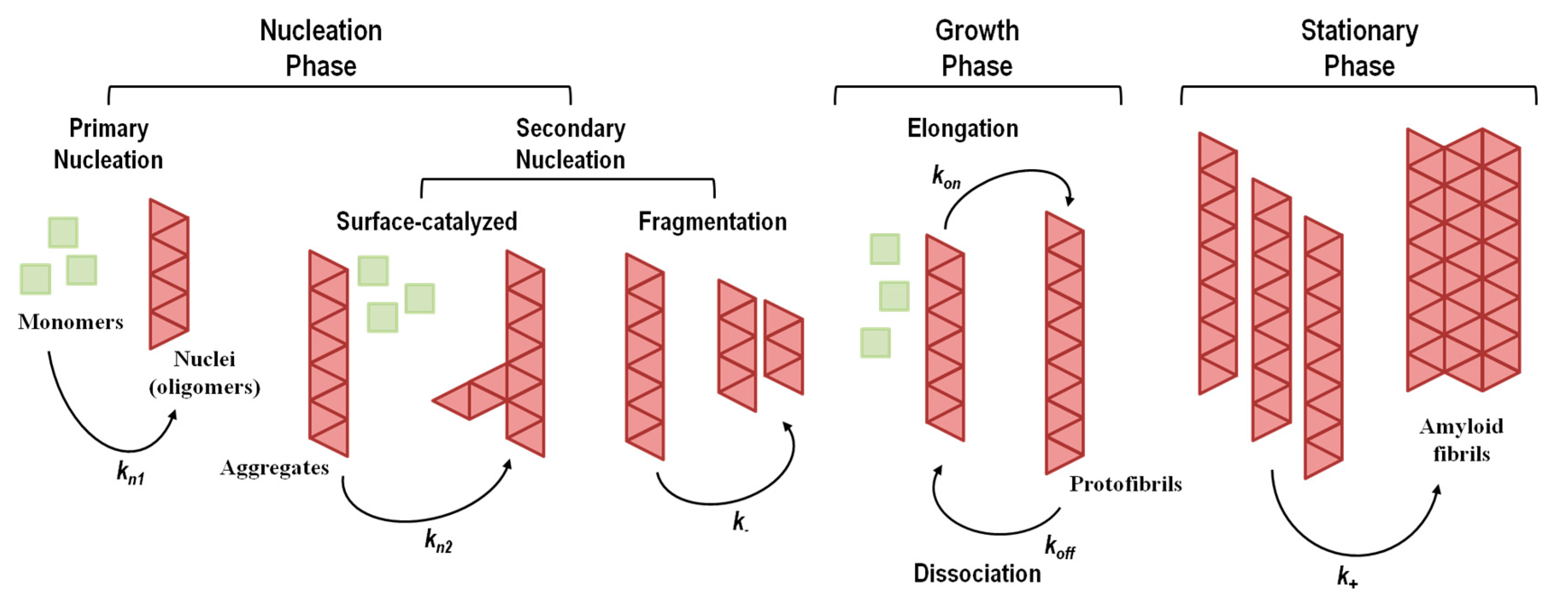
5. Kinetics and Thermodynamics of Amyloid Fibril Formation
| Nature of Monomeric Species | Description | References |
| Native monomer |
Normally folded proteins may retain a substantial tendency to aggregate through direct assembly of monomers in their native state when the native state exposes complementary surfaces. | [153][154][155][156] |
| Conformationally altered monomer | The native monomer has very low propensity to associate. Partial unfolding or conformational changes of the native monomer are required, resulting in a non-native species prone to aggregate. | [157][158][159][160][161][162] |
| Chemically modified monomer | Chemical modifications (deamidation, isomerization, hydrolysis, oxidation, photolysis, etc.) may cause conformational changes in native monomers, leading to species with high propensity to aggregate. | [163][164][165][166][167][168][169][170][171][172][173][174] |
| Nature of Aggregation Interfaces | Description | References |
| Gas-liquid interface |
Hydrophobic–hydrophilic interfaces may induce aggregation reactions. | [175][176][177][178][179][180][181] |
| Mechanical stress (agitation, stirring, pumping, or shaking) has been associated with cavitation which generates air bubbles and, consequently, the formation of an air-water interface which facilitates protein denaturation and aggregation. | [176][182][183][184][185][186][187][188][189] | |
| The use of beads during agitation accelerates the aggregation process by enhancing cavitation. | [190] | |
| Solid-liquid interface |
Solid-liquid interfaces may facilitate monomer encounters and initial monomer to monomer association and later further aggregation. | |
| In vitro, interaction with glass, silicone, graphite, polypropylene, Teflon, mica, gold, etc. might lead to protein partial unfolding and aggregation. | [181][191][192][193][194] | |
| In vitro and in vivo, flow through tubes and vessels produce shear forces that may lead to protein partial unfolding and aggregation. | [195] | |
| Freeze-thaw cycles create new ice-water interfaces which may induce protein partial unfolding and aggregation. | [189][191][196] | |
| Presence of metal ions, in particular, Cu2+ and Zn2+, may promote aggregation of protein monomers bearing metal-ion binding sites or binding residues (e.g., histidines). | [197][198][199][200][201] | |
| Monomer association at the surface of biomembranes or biomolecules may also enhance aggregation. | [202][203][204][205][206][207][208][209][210] |
5.1. Aggregation Via a Nucleation-Dependent Mechanism
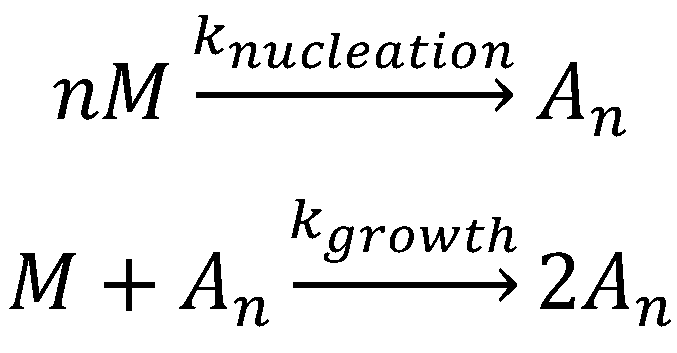
5.1.1. Primary Nucleation Mechanisms
5.1.2. Secondary Nucleation Mechanisms
5.2. Aggregation Via a Nucleation-Independent Mechanism
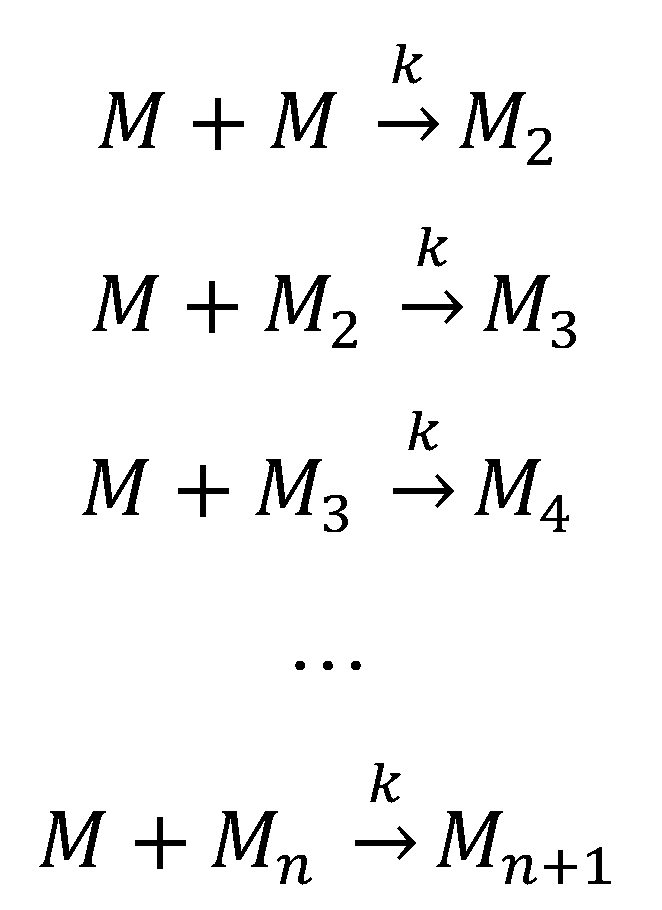
5.3. The Energy Landscape View of Protein Aggregation
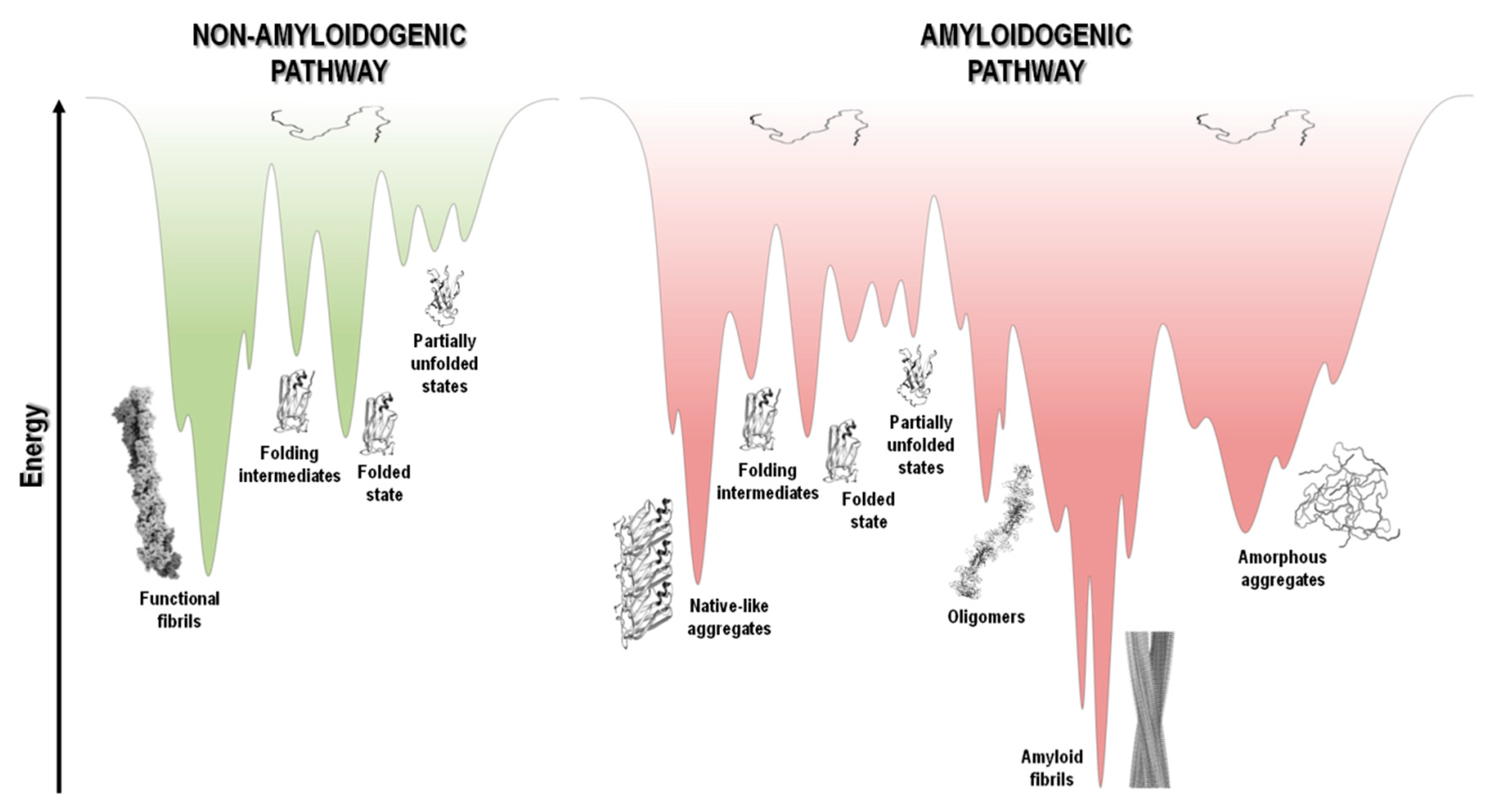
6. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/molecules25051195
References
- Fabrizio Chiti; Christopher M. Dobson; Protein Misfolding, Amyloid Formation, and Human Disease: A Summary of Progress Over the Last Decade. Annual Review of Biochemistry 2017, 86, 27-68, 10.1146/annurev-biochem-061516-045115.
- Matthew G. Iadanza; Matthew P. Jackson; Eric W. Hewitt; Neil A. Ranson; Sheena E. Radford; A new era for understanding amyloid structures and disease. Nature Reviews Molecular Cell Biology 2018, 19, 755-773, 10.1038/s41580-018-0060-8.
- Patterson, C. World Alzheimer Report 2018. The State of the Art of Dementia Research: New Frontiers; Alzheimer’s Disease International (ADI): London, UK, 2018; pp. 6–7.
- P. Westermark; K. Sletten; B. Johansson; G. G. Cornwell; Fibril in senile systemic amyloidosis is derived from normal transthyretin.. Proceedings of the National Academy of Sciences 1990, 87, 2843-2845, 10.1073/pnas.87.7.2843.
- Sanjay M. Banypersad; James C. Moon; Carol Whelan; Philip N. Hawkins; Ashutosh D. Wechalekar; Updates in Cardiac Amyloidosis: A Review. Journal of the American Heart Association 2012, 1, e000364, 10.1161/jaha.111.000364.
- Sipe, J.D. Amyloidosis. Annu. Rev. Biochem. 1992, 61, 947–975.
- Luis M. Blancas-Mejía; Marina Ramirez-Alvarado; Systemic Amyloidoses. Annual Review of Biochemistry 2013, 82, 745-774, 10.1146/annurev-biochem-072611-130030.
- Alexandre Quintas; Maria João Saraiva; Rui M.M Brito; The amyloidogenic potential of transthyretin variants correlates with their tendency to aggregate in solution. FEBS Letters 1997, 418, 297-300, 10.1016/s0014-5793(97)01398-7.
- Catarina S. H. Jesus; Zaida L. Almeida; Daniela C. Vaz; Tiago Q. Faria; Rui M. M. Brito; A New Folding Kinetic Mechanism for Human Transthyretin and the Influence of the Amyloidogenic V30M Mutation. International Journal of Molecular Sciences 2016, 17, 1428, 10.3390/ijms17091428.
- Kazufumi Takano; Jun Funahashi; Katsuhide Yutani; The stability and folding process of amyloidogenic mutant human lysozymes. JBIC Journal of Biological Inorganic Chemistry 2001, 268, 155-159, 10.1046/j.1432-1327.2001.01863.x.
- Rivka Leah Isaacson; Alan G. Weeds; Alan R. Fersht; Equilibria and kinetics of folding of gelsolin domain 2 and mutants involved in familial amyloidosis-Finnish type. Proceedings of the National Academy of Sciences 1999, 96, 11247-11252, 10.1073/pnas.96.20.11247.
- Marianne A. Grant; Noel Lazo; Aleksey Lomakin; Margaret M. Condron; Hiromi Arai; Ghiam Yamin; Alan C. Rigby; David B. Teplow; Familial Alzheimer's disease mutations alter the stability of the amyloid beta-protein monomer folding nucleus. Proceedings of the National Academy of Sciences 2007, 104, 16522-16527, 10.1073/pnas.0705197104.
- Christopher M. Dobson; Protein misfolding, evolution and disease. Trends in Biochemical Sciences 1999, 24, 329-332, 10.1016/s0968-0004(99)01445-0.
- Christopher M. Dobson; Protein folding and misfolding. Nature 2003, 426, 884-890, 10.1038/nature02261.
- Fabrizio Chiti; Christopher M. Dobson; Protein Misfolding, Functional Amyloid, and Human Disease. Annual Review of Biochemistry 2006, 75, 333-366, 10.1146/annurev.biochem.75.101304.123901.
- Douglas M. Fowler; Atanas V. Koulov; William E. Balch; Jeffery W. Kelly; Functional amyloid – from bacteria to humans. Trends in Biochemical Sciences 2007, 32, 217-224, 10.1016/j.tibs.2007.03.003.
- Chi L.L. Pham; Ann Kwan; Margaret Sunde; Functional amyloid: widespread in Nature, diverse in purpose. Essays in Biochemistry 2014, 56, 207-219, 10.1042/bse0560207.
- Daniel Otzen; Roland Riek; Functional Amyloids. Cold Spring Harbor Perspectives in Biology 2019, 11, a033860, 10.1101/cshperspect.a033860.
- Anamika Avni; Hema M. Swasthi; Anupa Majumdar; Samrat Mukhopadhyay; Intrinsically disordered proteins in the formation of functional amyloids from bacteria to humans. Progress in Molecular Biology and Translational Science 2019, 166, 109-143, 10.1016/bs.pmbts.2019.05.005.
- Jean D. Sipe; Alan S. Cohen; Review: History of the Amyloid Fibril. Journal of Structural Biology 2000, 130, 88-98, 10.1006/jsbi.2000.4221.
- O. Sumner Makin; Louise C. Serpell; Structures for amyloid fibrils. The FEBS Journal 2005, 272, 5950-5961, 10.1111/j.1742-4658.2005.05025.x.
- William Close; Matthias Neumann; Andreas Schmidt; Manuel Hora; Karthikeyan Annamalai; Matthias Schmidt; Bernd Reif; Volker Schmidt; Nikolaus Grigorieff; Marcus Fändrich; et al. Physical basis of amyloid fibril polymorphism. Nature Communications 2018, 9, 1-7, 10.1038/s41467-018-03164-5.
- Rebecca Nelson; Michael Sawaya; Melinda Balbirnie; Anders Østergaard Madsen; Christian Riekel; Robert Grothe; David Eisenberg; Structure of the cross-β spine of amyloid-like fibrils. Nature 2005, 435, 773-778, 10.1038/nature03680.
- Jalandoni-Buan, A.C.; Decena-Soliven, A.L.A.; Cao, E.P.; Barraquio, V.L.; Barraquio, W.L. Characterization and Identification of Congo Red Decolorizing Bacteria from Monocultures and Consortia. Philipp. J. Sci. 2010, 139, 71–78.
- Melanie R Nilsson; Techniques to study amyloid fibril formation in vitro. Methods 2004, 34, 151-160, 10.1016/j.ymeth.2004.03.012.
- William Klunk; Robert F. Jacob; R.Preston Mason; Quantifying Amyloid β-Peptide (Aβ) Aggregation Using the Congo Red-Aβ (CR–Aβ) Spectrophotometric Assay. Analytical Biochemistry 1999, 266, 66-76, 10.1006/abio.1998.2933.
- Robyn Eisert; Liseda Felau; Lesley R. Brown; Methods for enhancing the accuracy and reproducibility of Congo red and thioflavin T assays. Analytical Biochemistry 2006, 353, 144-146, 10.1016/j.ab.2006.03.015.
- Alexander J. Howie; Douglas B. Brewer; Optical properties of amyloid stained by Congo red: History and mechanisms. Micron 2008, 40, 285-301, 10.1016/j.micron.2008.10.002.
- Sait Sen; Gülçin Başdemir; Diagnosis of renal amyloidosis using Congo red fluorescence. Pathology International 2003, 53, 534-538, 10.1046/j.1440-1827.2003.01513.x.
- Tamar A. Giorgadze; Natsuko Shiina; Zubair W. Baloch; John E. Tomaszewski; Prabodh K. Gupta; Improved detection of amyloid in fat pad aspiration: An evaluation of Congo red stain by fluorescent microscopy. Diagnostic Cytopathology 2004, 31, 300-306, 10.1002/dc.20131.
- Harry Levine; Thioflavine T interaction with synthetic Alzheimer's diseaseβ-amyloid peptides: Detection of amyloid aggregation in solution. Protein Science 1993, 2, 404-410, 10.1002/pro.5560020312.
- Yair Porat; Adel Abramowitz; Ehud Gazit; Inhibition of Amyloid Fibril Formation by Polyphenols: Structural Similarity and Aromatic Interactions as a Common Inhibition Mechanism. Chemical Biology & Drug Design 2005, 67, 27-37, 10.1111/j.1747-0285.2005.00318.x.
- William G. Turnell; John T. Finch; Binding of the dye congo red to the amyloid protein pig insulin reveals a novel homology amongst amyloid-forming peptide sequences. Journal of Molecular Biology 1992, 227, 1205-1223, 10.1016/0022-2836(92)90532-o.
- Yong-Sung Kim; Theodore W. Randolph; Mark C. Manning; Fred J. Stevens; John F. Carpenter; Congo Red Populates Partially Unfolded States of an Amyloidogenic Protein to Enhance Aggregation and Amyloid Fibril Formation. Journal of Biological Chemistry 2003, 278, 10842-10850, 10.1074/jbc.m212540200.
- B Caughey; D Ernst; R E Race; Congo red inhibition of scrapie agent replication. Journal of Virology 1993, 67, 6270-6272, 10.1128/jvi.67.10.6270-6272.1993.
- A. Lorenzo; B. A. Yankner; Beta-amyloid neurotoxicity requires fibril formation and is inhibited by congo red.. Proceedings of the National Academy of Sciences 1994, 91, 12243-12247, 10.1073/pnas.91.25.12243.
- Harish Chander; Abha Chauhan; Ved Chauhan; Binding of Proteases to Fibrillar Amyloid-β Protein and its Inhibition by Congo Red. Journal of Alzheimer's Disease 2007, 12, 261-269, 10.3233/JAD-2007-12308.
- Vassar, P.S.; Culling, C.F. Fluorescent stains, with special reference to amyloid and connective tissues. Arch. Pathol. 1959, 68, 487–498.
- Kelényi, G. Thioflavin S fluorescent and Congo red anisotropic stainings in the histologic demonstration of amyloid. Acta Neuropathol. 1967, 7, 336–348.
- Nadine D. Younan; John H. Viles; A Comparison of Three Fluorophores for the Detection of Amyloid Fibers and Prefibrillar Oligomeric Assemblies. ThT (Thioflavin T); ANS (1-Anilinonaphthalene-8-sulfonic Acid); and bisANS (4,4′-Dianilino-1,1′-binaphthyl-5,5′-disulfonic Acid). Biochemistry 2015, 54, 4297-4306, 10.1021/acs.biochem.5b00309.
- Sureshbabu Nagarajan; Lisa J. Lapidus; Fluorescent Probe DCVJ Shows High Sensitivity for Characterization of Amyloid β-Peptide Early in the Lag Phase. ChemBioChem 2017, 18, 2205-2211, 10.1002/cbic.201700387.
- Rajesh Mishra; Daniel Sjölander; Per Hammarström; Spectroscopic characterization of diverse amyloid fibrils in vitro by the fluorescent dye Nile red. Mol. BioSyst. 2011, 7, 1232-1240, 10.1039/c0mb00236d.
- Vladyslava Kovalska; Svitlana Chernii; Mykhaylo Losytskyy; Iryna Tretyakova; Yan Dovbii; Alexandr Gorski; Victor Chernii; Rafal Czerwieniec; Sergiy Yarmoluk; Design of functionalized β-ketoenole derivatives as efficient fluorescent dyes for detection of amyloid fibrils. New Journal of Chemistry 2018, 42, 13308-13318, 10.1039/c8nj01020j.
- Adeline Marianne Fanni; Florencia A. Monge; Chia-Yu Lin; Arjun Thapa; Kiran Bhaskar; David G. Whitten; Eva Y. Chi; High Selectivity and Sensitivity of Oligomeric p-Phenylene Ethynylenes for Detecting Fibrillar and Prefibrillar Amyloid Protein Aggregates. ACS Chemical Neuroscience 2019, 10, 1813-1825, 10.1021/acschemneuro.8b00719.
- Sirvan Abbasbeigi; Hadi Adibi; Sajad Moradi; Seyyed Abolghasem Ghadami; Reza Khodarahmi; Detection/quantification of amyloid aggregation in solution using the novel fluorescent benzofuranone-derivative compounds as amyloid fluorescent probes: synthesis and in vitro characterization. Journal of the Iranian Chemical Society 2019, 16, 1225-1237, 10.1007/s13738-019-01599-1.
- Adam Crystal; Benoit I. Giasson; Alexander Crowe; Mei-Ping Kung; Zhi-Ping Zhuang; John Q. Trojanowski; Virginia M.-Y. Lee; A comparison of amyloid fibrillogenesis using the novel fluorescent compound K114. Journal of Neurochemistry 2003, 86, 1359-1368, 10.1046/j.1471-4159.2003.01949.x.
- Marie L. Schmidt; Theresa Schuck; Shelly Sheridan; Mei-Ping Kung; Hank Kung; Zhi-Ping Zhuang; Catherine Bergeron; Jacque S. Lamarche; Daniel Skovronsky; Benoit I. Giasson; et al. The Fluorescent Congo Red Derivative, (Trans, Trans)−1-Bromo-2,5-Bis-(3-Hydroxycarbonyl-4-Hydroxy)Styrylbenzene (BSB), Labels Diverse β-Pleated Sheet Structures in Postmortem Human Neurodegenerative Disease Brains. The American Journal of Pathology 2001, 159, 937-943, 10.1016/s0002-9440(10)61769-5.
- Scot D. Styren; Ronald L. Hamilton; Gisele C. Styren; William E. Klunk; X-34, A Fluorescent Derivative of Congo Red: A Novel Histochemical Stain for Alzheimer's Disease Pathology. Journal of Histochemistry & Cytochemistry 2000, 48, 1223-1232, 10.1177/002215540004800906.
- Xiao-Peng He; Qiong Deng; Liang Cai; Chang-Zheng Wang; Yi Zang; Jia Li; Guo-Rong Chen; He Tian; Fluorogenic Resveratrol-Confined Graphene Oxide For Economic and Rapid Detection Of Alzheimer’s Disease. ACS Applied Materials & Interfaces 2014, 6, 5379-5382, 10.1021/am5010909.
- K.D. Volkova; V.B. Kovalska; A.O. Balanda; Mykhaylo Losytskyy; A.G. Golub; R.J. Vermeij; Vinod Subramaniam; O.I. Tolmachev; S.M. Yarmoluk; Specific fluorescent detection of fibrillar α-synuclein using mono- and trimethine cyanine dyes. Bioorganic & Medicinal Chemistry 2008, 16, 1452-1459, 10.1016/j.bmc.2007.10.051.
- K.D. Volkova; V.B. Kovalska; A.O. Balanda; R.J. Vermeij; V. Subramaniam; Yu. L. Slominskii; S.M. Yarmoluk; Cyanine dye–protein interactions: Looking for fluorescent probes for amyloid structures. Journal of Biochemical and Biophysical Methods 2007, 70, 727-733, 10.1016/j.jbbm.2007.03.008.
- José Luna-Muñoz; Janneth Peralta-Ramirez; Laura Chávez-Macías; Charles R. Harrington; Claude M. Wischik; Raúl Mena; Thiazin red as a neuropathological tool for the rapid diagnosis of Alzheimer’s disease in tissue imprints. Acta Neuropathologica 2008, 116, 507-515, 10.1007/s00401-008-0431-x.
- Anna I. Sulatskaya; Maksim I. Sulatsky; Iuliia A. Antifeeva; Irina M. Kuznetsova; Konstantin K. Turoverov; Structural Analogue of Thioflavin T, DMASEBT, as a Tool for Amyloid Fibrils Study. Analytical Chemistry 2019, 91, 3131-3140, 10.1021/acs.analchem.8b05737.
- Matthew Biancalana; Shohei Koide; Molecular mechanism of Thioflavin-T binding to amyloid fibrils. Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics 2010, 1804, 1405-1412, 10.1016/j.bbapap.2010.04.001.
- Yakupova, E.I.; Bobyleva, L.G.; Vikhlyantsev, I.M.; Bobylev, A.G. Congo Red and amyloids: History and relationship. Biosci. Rep. 2019, 39.
- Merrill D. Benson; Joel N. Buxbaum; David S. Eisenberg; Giampaolo Merlini; Maria J. M. Saraiva; Yoshiki Sekijima; Jean D. Sipe; Per Westermark; Amyloid nomenclature 2018: recommendations by the International Society of Amyloidosis (ISA) nomenclature committee. Amyloid 2018, 25, 215-219, 10.1080/13506129.2018.1549825.
- A.J. Geddes; K.D. Parker; E.D.T. Atkins; E. Beighton; “Cross-β” conformation in proteins. Journal of Molecular Biology 1968, 32, 343-358, 10.1016/0022-2836(68)90014-4.
- M Sunde; C Blake; The Structure of Amyloid Fibrils by Electron Microscopy and X-Ray Diffraction. Advances in Protein Chemistry Volume 12 1997, 50, 123-159, 10.1016/s0065-3233(08)60320-4.
- Ravindra Kodali; Ronald Wetzel; Polymorphism in the intermediates and products of amyloid assembly. Current Opinion in Structural Biology 2007, 17, 48-57, 10.1016/j.sbi.2007.01.007.
- E. D. Eanes; G. G. Glenner; X-RAY DIFFRACTION STUDIES ON AMYLOID FILAMENTS. Journal of Histochemistry & Cytochemistry 1968, 16, 673-677, 10.1177/16.11.673.
- L. Bonar; A. S. Cohen; M. M. Skinner; Characterization of the Amyloid Fibril as a Cross- Protein. Experimental Biology and Medicine 1969, 131, 1373-1375, 10.3181/00379727-131-34110.
- Margaret Sunde; Louise Serpell; Mark Bartlam; Paul E Fraser; Mark B Pepys; Colin C.F Blake; Common core structure of amyloid fibrils by synchrotron X-ray diffraction. Journal of Molecular Biology 1997, 273, 729-739, 10.1006/jmbi.1997.1348.
- Rebecca Nelson; David Eisenberg; Recent atomic models of amyloid fibril structure. Current Opinion in Structural Biology 2006, 16, 260-265, 10.1016/j.sbi.2006.03.007.
- M. I. Ivanova; M. J. Thompson; D. Eisenberg; A systematic screen of beta2-microglobulin and insulin for amyloid-like segments. Proceedings of the National Academy of Sciences 2006, 103, 4079-4082, 10.1073/pnas.0511298103.
- David S. Eisenberg; Michael R. Sawaya; Structural Studies of Amyloid Proteins at the Molecular Level. Annual Review of Biochemistry 2017, 86, 69-95, 10.1146/annurev-biochem-061516-045104.
- Shilpa Sambashivan; Yanshun Liu; Michael Sawaya; Mari Gingery; David Eisenberg; Amyloid-like fibrils of ribonuclease A with three-dimensional domain-swapped and native-like structure. Nature 2005, 437, 266-269, 10.1038/nature03916.
- Marcus Fändrich; Jessica Meinhardt; Nikolaus Grigorieff; Structural polymorphism of Alzheimer Aβ and other amyloid fibrils. Prion 2009, 3, 89-93, 10.4161/pri.3.2.8859.
- Jessica Meinhardt; Carsten Sachse; Peter Hortschansky; Nikolaus Grigorieff; Marcus Fändrich; Aβ(1-40) Fibril Polymorphism Implies Diverse Interaction Patterns in Amyloid Fibrils. Journal of Molecular Biology 2009, 386, 869-877, 10.1016/j.jmb.2008.11.005.
- Karolina L. Zapadka; Frederik J. Becher; A. L. Gomes Dos Santos; Sophie E. Jackson; Factors affecting the physical stability (aggregation) of peptide therapeutics. Interface Focus 2017, 7, 20170030-20170030, 10.1098/rsfs.2017.0030.
- AGGRESCAN: a server for the prediction and evaluation of . , , , .
- Natalia S. de Groot; Virginia Castillo; Ricardo Graña-Montes; Salvador Ventura; AGGRESCAN: Method, Application, and Perspectives for Drug Design. Methods in Molecular Biology 2011, 819, 199-220, 10.1007/978-1-61779-465-0_14.
- Rafael Zambrano; Michal Jamroz; Agata Szczasiuk; Jordi Pujols; Sebastian Kmiecik; Salvador Ventura; AGGRESCAN3D (A3D): server for prediction of aggregation properties of protein structures. Nucleic Acids Research 2015, 43, W306-W313, 10.1093/nar/gkv359.
- Jordi Pujols; Samuel Peña-Díaz; Salvador Ventura; AGGRESCAN3D: Toward the Prediction of the Aggregation Propensities of Protein Structures. Mitochondrial Bioenergetics 2018, 1762, 427-443, 10.1007/978-1-4939-7756-7_21.
- Pawel P. Wozniak; Malgorzata Kotulska; AmyLoad: website dedicated to amyloidogenic protein fragments. Bioinformatics 2015, 31, 3395-3397, 10.1093/bioinformatics/btv375.
- Burdukiewicz, M.; Sobczyk, P.; Rödiger, S.; Duda-Madej, A.; Mackiewicz, P.; Kotulska, M. Amyloidogenic motifs revealed by n-gram analysis. Sci. Rep. 2017, 7, 12961.
- Charles W. O'Donnell; Jérôme Waldispühl; Mieszko Lis; Randal Halfmann; Srinivas Devadas; Susan Lindquist; Bonnie Berger; A method for probing the mutational landscape of amyloid structure. Bioinformatics 2011, 27, i34-i42, 10.1093/bioinformatics/btr238.
- Kimon K Frousios; Vassiliki A Iconomidou; Carolina-Maria Karletidi; Stavros J Hamodrakas; Amyloidogenic determinants are usually not buried. BMC Structural Biology 2009, 9, 44-44, 10.1186/1472-6807-9-44.
- Antonios Tsolis; Nikos Papandreou; Vassiliki A. Iconomidou; Stavros J. Hamodrakas; A Consensus Method for the Prediction of ‘Aggregation-Prone’ Peptides in Globular Proteins. PLOS ONE 2013, 8, e54175, 10.1371/journal.pone.0054175.
- Carlos Familia; Sarah R. Dennison; Alexandre Quintas; David A. Phoenix; Prediction of Peptide and Protein Propensity for Amyloid Formation. PLoS ONE 2015, 10, e0134679, 10.1371/journal.pone.0134679.
- Allen W. Bryan; Matthew Menke; Lenore J. Cowen; Susan L. Lindquist; Bonnie Berger; BETASCAN: Probable β-amyloids Identified by Pairwise Probabilistic Analysis. PLoS Computational Biology 2009, 5, e1000333, 10.1371/journal.pcbi.1000333.
- Melissa J. Landrum; Jennifer M. Lee; Mark Benson; Garth Brown; Chen Chao; Shanmuga Chitipiralla; Baoshan Gu; Jennifer Hart; Uglas Hoffman; Jeffrey Hoover; et al. ClinVar: public archive of interpretations of clinically relevant variants. Nucleic Acids Research 2015, 44, D862-D868, 10.1093/nar/gkv1222 [doi].
- Hamed Tabatabaei Ghomi; Elizabeth M. Topp; Markus A. Lill; Fibpredictor: a computational method for rapid prediction of amyloid fibril structures. Journal of Molecular Modeling 2016, 22, 206, 10.1007/s00894-016-3066-1.
- Pawel Gasior; Malgorzata Kotulska; FISH Amyloid – a new method for finding amyloidogenic segments in proteins based on site specific co-occurence of aminoacids. BMC Bioinformatics 2014, 15, 54-54, 10.1186/1471-2105-15-54.
- Sergiy Garbuzynskiy; Michail Yu. Lobanov; Oxana V. Galzitskaya; FoldAmyloid: a method of prediction of amyloidogenic regions from protein sequence. Bioinformatics 2009, 26, 326-332, 10.1093/bioinformatics/btp691.
- A. Mary Thangakani; Sandeep Kumar; R. Nagarajan; D. Velmurugan; M. Michael Gromiha; GAP: towards almost 100 percent prediction for β-strand-mediated aggregating peptides with distinct morphologies. Bioinformatics 2014, 30, 1983-1990, 10.1093/bioinformatics/btu167.
- Taner Z. Sen; Robert L. Jernigan; Jean Garnier; Andrzej Kloczkowski; GOR V server for protein secondary structure prediction. Bioinformatics 2005, 21, 2787-2788, 10.1093/bioinformatics/bti408.
- Maksim Kouza; Eshel Faraggi; Andrzej Kolinski; Andrzej Kloczkowski; The GOR Method of Protein Secondary Structure Prediction and Its Application as a Protein Aggregation Prediction Tool. Springer Protocols Handbooks 2016, 1484, 7-24, 10.1007/978-1-4939-6406-2_2.
- Mathieu Emily; Anthony Talvas; Christian Delamarche; MetAmyl: A METa-Predictor for AMYLoid Proteins. PLoS ONE 2013, 8, e79722, 10.1371/journal.pone.0079722.
- Farzeen Munir; Sadaf Gull; Amina Asif; Fayyaz-Ul-Amir Afsar Minhas; MILAMP: Multiple Instance Prediction of Amyloid Proteins. IEEE/ACM Transactions on Computational Biology and Bioinformatics 2019, 18, 1142-1150, 10.1109/tcbb.2019.2936846.
- Changsik Kim; Jiwon Choi; Seong Joon Lee; William J. Welsh; Sukjoon Yoon; NetCSSP: web application for predicting chameleon sequences and amyloid fibril formation. Nucleic Acids Research 2009, 37, W469-W473, 10.1093/nar/gkp351.
- Antonio Trovato; Fabrizio Chiti; Amos Maritan; Flavio Seno; Insight into the Structure of Amyloid Fibrils from the Analysis of Globular Proteins. PLOS Computational Biology 2006, 2, e170, 10.1371/journal.pcbi.0020170.
- Antonio Trovato; Flavio Seno; Silvio C.E. Tosatto; The PASTA server for protein aggregation prediction. Protein Engineering Design and Selection 2007, 20, 521-523, 10.1093/protein/gzm042.
- Ian Walsh; Flavio Seno; Silvio C.E. Tosatto; Antonio Trovato; PASTA 2.0: an improved server for protein aggregation prediction. Nucleic Acids Research 2014, 42, W301-W307, 10.1093/nar/gku399.
- Mengting Niu; Yanjuan Li; Chunyu Wang; Ke Han; RFAmyloid: A Web Server for Predicting Amyloid Proteins. International Journal of Molecular Sciences 2018, 19, 2071, 10.3390/ijms19072071.
- G. De Baets; J. Van Durme; Joke Reumers; Sebastian Maurer-Stroh; P. Vanhee; J. Dopazo; J. Schymkowitz; F. Rousseau; SNPeffect 4.0: on-line prediction of molecular and structural effects of protein-coding variants. Nucleic Acids Research 2011, 40, D935-D939, 10.1093/nar/gkr996.
- Allen W. Bryan Jr.; Colm O'Donnell; Matthew Menke; Lenore J. Cowen; Susan Lindquist; Bonnie Berger; STITCHER: Dynamic assembly of likely amyloid and prion β‐structures from secondary structure predictions. Proteins: Structure, Function, and Bioinformatics 2011, 80, 410-420, 10.1002/prot.23203.
- Ana-Maria Fernandez-Escamilla; Frederic Rousseau; Joost Schymkowitz; Luis Serrano; Prediction of sequence-dependent and mutational effects on the aggregation of peptides and proteins. Nature Biotechnology 2004, 22, 1302-1306, 10.1038/nbt1012 nbt1012 [pii].
- Sebastian Maurer-Stroh; Maja Debulpaep; Nico Kuemmerer; Manuela Lopez De La Paz; Ivo Martins; Joke Reumers; Kyle L Morris; Alastair Copland; Louise Serpell; Luis Serrano; et al. Exploring the sequence determinants of amyloid structure using position-specific scoring matrices. Nature Methods 2010, 7, 237-242, 10.1038/nmeth.1432.
- Jacinte Beerten; Joost Van Durme; Rodrigo Gallardo; Emidio Capriotti; Louise Serpell; Frederic Rousseau; Joost Schymkowitz; WALTZ-DB: a benchmark database of amyloidogenic hexapeptides. Bioinformatics 2015, 31, 1698-1700, 10.1093/bioinformatics/btv027.
- Nikolaos Louros; Katerina Konstantoulea; Matthias De Vleeschouwer; Meine Ramakers; Joost Schymkowitz; Frederic Rousseau; WALTZ-DB 2.0: an updated database containing structural information of experimentally determined amyloid-forming peptides. Nucleic Acids Research 2019, 48, D389-D393, 10.1093/nar/gkz758.
- Michael J. Thompson; Stuart A. Sievers; John Karanicolas; Magdalena I. Ivanova; David Baker; David Eisenberg; The 3D profile method for identifying fibril-forming segments of proteins. Proceedings of the National Academy of Sciences 2006, 103, 4074-4078, 10.1073/pnas.0511295103.
- Gian Gaetano Tartaglia; Michele Vendruscolo; The Zyggregator method for predicting protein aggregation propensities. Chemical Society Reviews 2008, 37, 1395-401, 10.1039/b706784b.
- Michael GroB; Proteins that Convert from a Helix to b Sheet Implications for Folding and Disease. Current Protein & Peptide Science 2000, 1, 339-347, 10.2174/1389203003381289.
- Charlotte Hauser; R. Deng; A. Mishra; Y. Loo; U. Khoe; F. Zhuang; D. W. Cheong; A. Accardo; Michael Sullivan; C. Riekel; et al. Natural tri- to hexapeptides self-assemble in water to amyloid -type fiber aggregates by unexpected -helical intermediate structures. Proceedings of the National Academy of Sciences 2011, 108, 1361-1366, 10.1073/pnas.1014796108.
- Dhiman Ghosh; Pradeep K. Singh; Shruti Sahay; Narendra Nath Jha; Reeba Jacob; Shamik Sen; Ashutosh Kumar; Roland Riek; Samir K. Maji; Structure based aggregation studies reveal the presence of helix-rich intermediate during α-Synuclein aggregation. Scientific Reports 2015, 5, 9228-9228, 10.1038/srep09228.
- Katarzyna Cieślik-Boczula; Alpha-helix to beta-sheet transition in long-chain poly- l -lysine: Formation of alpha-helical fibrils by poly- l -lysine. Biochimie 2017, 137, 106-114, 10.1016/j.biochi.2017.03.006.
- Ming Ni; Shuangmu Zhuo; Ciprian Iliescu; Peter T. C. So; Jodhbir S. Mehta; Hanry Yu; Charlotte A. E. Hauser; Self‐assembling amyloid‐like peptides as exogenous second harmonic probes for bioimaging applications. Journal of Biophotonics 2019, 12, e201900065, 10.1002/jbio.201900065.
- Einav Tayeb-Fligelman; Orly Tabachnikov; Asher Moshe; Orit Goldshmidt-Tran; Michael R. Sawaya; Nicolas Coquelle; Jacques-Philippe Colletier; Meytal Landau; The cytotoxic Staphylococcus aureus PSMα3 reveals a cross-α amyloid-like fibril. Science 2017, 355, 831-833, 10.1126/science.aaf4901.
- Luc Bousset; Neil Thomson; Sheena E. Radford; Ronald Melki; The yeast prion Ure2p retains its native α-helical conformation upon assembly into protein fibrils in vitro. The EMBO Journal 2002, 21, 2903-2911, 10.1093/emboj/cdf303.
- K. S. Taylor; M.-Z. Lou; T.-M. Chin; N. C. Yang; R. M. Garavito; A novel, multilayer structure of a helical peptide. Protein Science 1996, 5, 414-421, 10.1002/pro.5560050302.
- Gilbert G. Privé; Daniel H. Anderson; Laura Wesson; Duilio Cascio; David Eisenberg; Packed protein bilayers in the 0.90 å resolution structure of a designed alpha helical bundle. Protein Science 1999, 8, 1400-1409, 10.1110/ps.8.7.1400.
- Sudipta Mondal; Lihi Adler-Abramovich; Ayala Lampel; Yaron Bram; Sophia Lipstman; Ehud Gazit; Formation of functional super-helical assemblies by constrained single heptad repeat. Nature Communications 2015, 6, 8615, 10.1038/ncomms9615.
- Tj Brunette; Fabio Parmeggiani; Po-Ssu Huang; Gira Bhabha; Damian C. Ekiert; Susan E. Tsutakawa; Greg L. Hura; John A. Tainer; David Baker; Exploring the repeat protein universe through computational protein design. Nature 2015, 528, 580-584, 10.1038/nature16162.
- Pham, C.L.; Shanmugam, N.; Strange, M.; O’Carroll, A.; Brown, J.W.; Sierecki, E.; Gambin, Y.; Steain, M.; Sunde, M. Viral M45 and necroptosis-associated proteins form heteromeric amyloid assemblies. EMBO Rep. 2019, 20.
- Miguel Mompeán; Wenbo Li; Jixi Li; Ségolène Laage; Ansgar B. Siemer; Gunes Bozkurt; Hao Wu; Ann E. McDermott; The Structure of the Necrosome RIPK1-RIPK3 Core, a Human Hetero-Amyloid Signaling Complex. Cell 2018, 173, 1244-1253.e10, 10.1016/j.cell.2018.03.032.
- Brian O'Nuallain; Angela D. Williams; Per Westermark; Ronald Wetzel; Seeding Specificity in Amyloid Growth Induced by Heterologous Fibrils. Journal of Biological Chemistry 2004, 279, 17490-17499, 10.1074/jbc.m311300200.
- Marie E. Oskarsson; Johan F Paulsson; Sebastian W. Schultz; Martin Ingelsson; Per Westermark; Gunilla T. Westermark; In Vivo Seeding and Cross-Seeding of Localized Amyloidosis. The American Journal of Pathology 2015, 185, 834-846, 10.1016/j.ajpath.2014.11.016.
- Rodrigo Morales; Lisbell D. Estrada; Rodrigo Diaz-Espinoza; Diego Morales-Scheihing; Maria C. Jara; Joaquin Castilla; Claudio Soto; Molecular Cross Talk between Misfolded Proteins in Animal Models of Alzheimer's and Prion Diseases. The Journal of Neuroscience 2010, 30, 4528-4535, 10.1523/JNEUROSCI.5924-09.2010.
- Janett Köppen; Anja Schulze; Lisa Machner; Michael Wermann; Rico Eichentopf; Max Guthardt; Angelika Hähnel; Jessica Klehm; Marie-Christin Kriegeskorte; Maike Hartlage-Rübsamen; et al. Amyloid-Beta Peptides Trigger Aggregation of Alpha-Synuclein In Vitro. Molecules 2020, 25, 580, 10.3390/molecules25030580.
- Gotz, J.; Chen, F.; van Dorpe, J.; Nitsch, R.M. Formation of Neurofibrillary Tangles in P301L Tau Transgenic Mice Induced by Abeta 42 Fibrils. Science 2001, 293, 1491–1495.
- Cristian A. Lasagna-Reeves; Diana L. Castillo-Carranza; Marcos J. Guerrero-Muñoz; George R. Jackson; Rakez Kayed; Preparation and Characterization of Neurotoxic Tau Oligomers. Biochemistry 2010, 49, 10039-10041, 10.1021/bi1016233.
- Karishma Bhasne; Sanjana Sebastian; Neha Jain; Samrat Mukhopadhyay; Synergistic Amyloid Switch Triggered by Early Heterotypic Oligomerization of Intrinsically Disordered α-Synuclein and Tau. Journal of Molecular Biology 2018, 430, 2508-2520, 10.1016/j.jmb.2018.04.020.
- K. Lundmark; G. T. Westermark; A. Olsen; P. Westermark; Protein fibrils in nature can enhance amyloid protein A amyloidosis in mice: Cross-seeding as a disease mechanism. Proceedings of the National Academy of Sciences 2005, 102, 6098-6102, 10.1073/pnas.0501814102.
- I. L. Derkatch; S. M. Uptain; T. F. Outeiro; Rajaraman Krishnan; S. L. Lindquist; S. W. Liebman; Effects of Q/N-rich, polyQ, and non-polyQ amyloids on the de novo formation of the [PSI+] prion in yeast and aggregation of Sup35 in vitro. Proceedings of the National Academy of Sciences 2004, 101, 12934-12939, 10.1073/pnas.0404968101.
- Neal Hammer; J. C. Schmidt; M. R. Chapman; The curli nucleator protein, CsgB, contains an amyloidogenic domain that directs CsgA polymerization. Proceedings of the National Academy of Sciences 2007, 104, 12494-12499, 10.1073/pnas.0703310104.
- Kyle L. Morris; Louise C. Serpell; X-Ray Fibre Diffraction Studies of Amyloid Fibrils. Springer Protocols Handbooks 2012, 849, 121-135, 10.1007/978-1-61779-551-0_9.
- Robert Tycko; Solid-State NMR Studies of Amyloid Fibril Structure. Annual Review of Physical Chemistry 2011, 62, 279-299, 10.1146/annurev-physchem-032210-103539.
- Louise C Serpell; Judith M Smith; Direct visualisation of the β-sheet structure of synthetic Alzheimer’s amyloid. Journal of Molecular Biology 2000, 299, 225-231, 10.1006/jmbi.2000.3650.
- Rachel W. Martin; John E. Kelly; Jessica I. Kelz; Advances in instrumentation and methodology for solid-state NMR of biological assemblies. Journal of Structural Biology 2018, 206, 73-89, 10.1016/j.jsb.2018.09.003.
- Beat H. Meier; Roland Riek; Anja Böckmann; Emerging Structural Understanding of Amyloid Fibrils by Solid-State NMR. Trends in Biochemical Sciences 2017, 42, 777-787, 10.1016/j.tibs.2017.08.001.
- Xiao-Chen Bai; Greg McMullan; Sjors H.W Scheres; How cryo-EM is revolutionizing structural biology. Trends in Biochemical Sciences 2015, 40, 49-57, 10.1016/j.tibs.2014.10.005.
- Alan S. Cohen; Evan Calkins; Electron Microscopic Observations on a Fibrous Component in Amyloid of Diverse Origins. Nature 1959, 183, 1202-1203, 10.1038/1831202a0.
- Werner Kühlbrandt; The Resolution Revolution. Science 2014, 343, 1443-1444, 10.1126/science.1251652.
- Lilia Milanesi; Tania Sheynis; Wei-Feng Xue; Elena Orlova; Andrew Hellewell; Raz Jelinek; Eric Hewitt; Sheena Radford; Helen R. Saibil; Direct three-dimensional visualization of membrane disruption by amyloid fibrils. Proceedings of the National Academy of Sciences 2012, 109, 20455-20460, 10.1073/pnas.1206325109.
- Kevin W. Tipping; Patricija van Oosten-Hawle; Eric Hewitt; Sheena E. Radford; Amyloid Fibres: Inert End-Stage Aggregates or Key Players in Disease?. Trends in Biochemical Sciences 2015, 40, 719-727, 10.1016/j.tibs.2015.10.002.
- Vibha Taneja; Meenakshi Verma; Abhishek Vats; Toxic species in amyloid disorders: Oligomers or mature fibrils. Annals of Indian Academy of Neurology 2015, 18, 138-45, 10.4103/0972-2327.144284.
- Elisa Evangelisti; Roberta Cascella; Matteo Becatti; Giovanna Marrazza; Christopher M. Dobson; Fabrizio Chiti; Massimo Stefani; Cristina Cecchi; Binding affinity of amyloid oligomers to cellular membranes is a generic indicator of cellular dysfunction in protein misfolding diseases. Scientific Reports 2016, 6, 32721, 10.1038/srep32721.
- Mirella Vivoli Vega; Roberta Cascella; Serene W Chen; Giuliana Fusco; Alfonso De Simone; Christopher M. Dobson; Cristina Cecchi; Fabrizio Chiti; The Toxicity of Misfolded Protein Oligomers Is Independent of Their Secondary Structure. ACS Chemical Biology 2019, 14, 1593-1600, 10.1021/acschembio.9b00324.
- Heidi Olzscha; Sonya M. Schermann; Andreas C. Woerner; Stefan Pinkert; Michael H. Hecht; Gian G. Tartaglia; Michele Vendruscolo; Manajit Hayer-Hartl; F. Ulrich Hartl; R. Martin Vabulas; et al. Amyloid-like Aggregates Sequester Numerous Metastable Proteins with Essential Cellular Functions. Cell 2011, 144, 67-78, 10.1016/j.cell.2010.11.050.
- Benedetta Mannini; Estefania Mulvihill; Caterina Sgromo; Roberta Cascella; Reza Khodarahmi; Matteo Ramazzotti; Christopher M. Dobson; Cristina Cecchi; Fabrizio Chiti; Toxicity of Protein Oligomers Is Rationalized by a Function Combining Size and Surface Hydrophobicity. ACS Chemical Biology 2014, 9, 2309-2317, 10.1021/cb500505m.
- Benedetta Mannini; R. Cascella; M. Zampagni; M. van Waarde-Verhagen; S. Meehan; Cintia Roodveldt; S. Campioni; M. Boninsegna; A. Penco; A. Relini; et al. Molecular mechanisms used by chaperones to reduce the toxicity of aberrant protein oligomers. Proceedings of the National Academy of Sciences 2012, 109, 12479-12484, 10.1073/pnas.1117799109.
- Monica Bucciantini; Elisa Giannoni; Fabrizio Chiti; Fabiana Baroni; Lucia Formigli; Jesús Zurdo; Niccolò Taddei; Giampietro Ramponi; Christopher M. Dobson; Massimo Stefani; et al. Inherent toxicity of aggregates implies a common mechanism for protein misfolding diseases. Nature 2002, 416, 507-511, 10.1038/416507a.
- Joanne McLaurin; Avi Chakrabartty; Membrane Disruption by Alzheimer β-Amyloid Peptides Mediated through Specific Binding to Either Phospholipids or Gangliosides. Journal of Biological Chemistry 1996, 271, 26482-26489, 10.1074/jbc.271.43.26482.
- Iryna Benilova; Eric Karran; Bart De Strooper; The toxic Aβ oligomer and Alzheimer's disease: an emperor in need of clothes. Nature Neuroscience 2012, 15, 349-357, 10.1038/nn.3028.
- Sophia C. Goodchild; Tania Sheynis; Rebecca Thompson; Kevin W. Tipping; Wei-Feng Xue; Neil A. Ranson; Paul A. Beales; Eric W. Hewitt; Sheena E. Radford; β2-Microglobulin Amyloid Fibril-Induced Membrane Disruption Is Enhanced by Endosomal Lipids and Acidic pH. PLOS ONE 2014, 9, e104492, 10.1371/journal.pone.0104492.
- Konstanze F. Winklhofer; Christian Haass; Mitochondrial dysfunction in Parkinson's disease. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease 2010, 1802, 29-44, 10.1016/j.bbadis.2009.08.013.
- Hazel L. Roberts; David R. Brown; Seeking a Mechanism for the Toxicity of Oligomeric α-Synuclein. Biomolecules 2015, 5, 282-305, 10.3390/biom5020282.
- Thomas C.T. Michaels; Anđela Šarić; Johnny Habchi; Sean Chia; Georg Meisl; Michele Vendruscolo; Christopher M. Dobson; Tuomas P.J. Knowles; Chemical Kinetics for Bridging Molecular Mechanisms and Macroscopic Measurements of Amyloid Fibril Formation. Annual Review of Physical Chemistry 2018, 69, 273-298, 10.1146/annurev-physchem-050317-021322.
- Carl Frieden; Protein aggregation processes: In search of the mechanism. Protein Science 2007, 16, 2334-2344, 10.1110/ps.073164107.
- Alexander K. Buell; Christopher M. Dobson; Tuomas P.J. Knowles; The physical chemistry of the amyloid phenomenon: thermodynamics and kinetics of filamentous protein aggregation. Essays in Biochemistry 2014, 56, 11-39, 10.1042/bse0560011.
- Joseph T. Jarrett; Jr. Peter T. Lansbury; Amyloid fibril formation requires a chemically discriminating nucleation event: studies of an amyloidogenic sequence from the bacterial protein OsmB. Biochemistry 1992, 31, 12345-12352, 10.1021/bi00164a008.
- Walraj S. Gosal; Isobel J. Morten; Eric W. Hewitt; D. Alastair Smith; Neil H. Thomson; Sheena E. Radford; Competing Pathways Determine Fibril Morphology in the Self-assembly of β2-Microglobulin into Amyloid. Journal of Molecular Biology 2005, 351, 850-864, 10.1016/j.jmb.2005.06.040.
- Gemma Soldi; Francesco Bemporad; Silvia Torrassa; Annalisa Relini; Matteo Ramazzotti; Niccolò Taddei; Fabrizio Chiti; Amyloid Formation of a Protein in the Absence of Initial Unfolding and Destabilization of the Native State. Biophysical Journal 2005, 89, 4234-4244, 10.1529/biophysj.105.067538.
- Georgia Plakoutsi; Francesco Bemporad; Martino Calamai; Niccolò Taddei; Christopher M. Dobson; Fabrizio Chiti; Evidence for a Mechanism of Amyloid Formation Involving Molecular Reorganisation within Native-like Precursor Aggregates. Journal of Molecular Biology 2005, 351, 910-922, 10.1016/j.jmb.2005.06.043.
- Francesco Bemporad; Tommaso Vannocci; Lorena Varela; Ana I. Azuaga; Fabrizio Chiti; A model for the aggregation of the acylphosphatase from Sulfolobus solfataricus in its native-like state. Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics 2008, 1784, 1986-1996, 10.1016/j.bbapap.2008.08.021.
- Javier Garcia-Pardo; Ricardo Graña-Montes; Marc Fernandez-Mendez; Angels Ruyra; Nerea Roher; Francesc Xavier Aviles; Julia Lorenzo; Salvador Ventura; Amyloid Formation by Human Carboxypeptidase D Transthyretin-like Domain under Physiological Conditions. Journal of Biological Chemistry 2014, 289, 33783-33796, 10.1074/jbc.m114.594804.
- Stephen W. Raso; Jeff Abel; Jesse M. Barnes; Kevin M. Maloney; Gary Pipes; Michael J. Treuheit; Jonathan King; David N. Brems; Aggregation of granulocyte-colony stimulating factor in vitro involves a conformationally altered monomeric state. Protein Science 2005, 14, 2246-2257, 10.1110/ps.051489405.
- Brent S. Kendrick; John F. Carpenter; Jeffrey L. Cleland; Theodore W. Randolph; A transient expansion of the native state precedes aggregation of recombinant human interferon. Proceedings of the National Academy of Sciences 1998, 95, 14142-14146, 10.1073/pnas.95.24.14142.
- Alexandre Quintas; Maria João Saraiva; Rui M. M. Brito; The Tetrameric Protein Transthyretin Dissociates to a Non-native Monomer in Solution. Journal of Biological Chemistry 1999, 274, 32943-32949, 10.1074/jbc.274.46.32943.
- Alexandre Quintas; Daniela Barroso De Moura Cipreste Vaz; Isabel Cardoso; Maria João Saraiva; Rui Brito; Tetramer Dissociation and Monomer Partial Unfolding Precedes Protofibril Formation in Amyloidogenic Transthyretin Variants. Journal of Biological Chemistry 2001, 276, 27207-27213, 10.1074/jbc.m101024200.
- David R. Booth; Margaret Sunde; Vittorio Bellotti; Carol V. Robinson; Winston L. Hutchinson; Paul E. Fraser; Philip N. Hawkins; Christopher M. Dobson; Sheena Radford; Colin C. F. Blake; et al. Instability, unfolding and aggregation of human lysozyme variants underlying amyloid fibrillogenesis. Nature 1997, 385, 787-793, 10.1038/385787a0.
- J. Iñaki Guijarro; Margaret Sunde; Jonathan Jones; Iain D. Campbell; Christopher M. Dobson; Amyloid fibril formation by an SH3 domain. Proceedings of the National Academy of Sciences 1998, 95, 4224-4228, 10.1073/pnas.95.8.4224.
- Truscott, R.J.W.; Augusteyn, R.C. Oxidative changes in human lens proteins during senile nuclear cataract formation. Biochim. Et Biophys. Acta (BBA)-Protein Struct. 1977, 492, 43–52.
- Purnananda Guptasarma; Dorairajan Balasubramanian; Seichii Matsugo; Isao Saito; Hydroxyl radical mediated damage to proteins, with special reference to the crystallins. Biochemistry 1992, 31, 4296-4303, 10.1021/bi00132a021.
- Suzanne Hermeling; Huub Schellekens; Coen Maas; Martijn F.B.G. Gebbink; Daan J.A. Crommelin; Wim Jiskoot; Antibody Response to Aggregated Human Interferon Alpha2b in Wild-type and Transgenic Immune Tolerant Mice Depends on Type and Level of Aggregation. Journal of Pharmaceutical Sciences 2006, 95, 1084-1096, 10.1002/jps.20599.
- Hamid Mirzaei; Fred Regnier; Protein:protein aggregation induced by protein oxidation. Journal of Chromatography B 2008, 873, 8-14, 10.1016/j.jchromb.2008.04.025.
- Christian Landles; Kirupa Sathasivam; Andreas Weiss; Ben Woodman; Hilary Moffitt; Steve Finkbeiner; Banghua Sun; Juliette Gafni; Lisa M. Ellerby; Yvon Trottier; et al. Proteolysis of Mutant Huntingtin Produces an Exon 1 Fragment That Accumulates as an Aggregated Protein in Neuronal Nuclei in Huntington Disease. Journal of Biological Chemistry 2010, 285, 8808-8823, 10.1074/jbc.m109.075028.
- Gary K.L. Chan; Andrzej Witkowski; Donald L. Gantz; Tianqi O. Zhang; Martin T. Zanni; Shobini Jayaraman; Giorgio Cavigiolio; Myeloperoxidase-mediated Methionine Oxidation Promotes an Amyloidogenic Outcome for Apolipoprotein A-I. Journal of Biological Chemistry 2015, 290, 10958-10971, 10.1074/jbc.m114.630442.
- M. A. Rosenfeld; V. B. Leonova; M. L. Konstantinova; S. D. Razumovskii; Self-assembly of fibrin monomers and fibrinogen aggregation during ozone oxidation. Biochemistry (Moscow) 2009, 74, 41-46, 10.1134/s0006297909010064.
- Alireza Roostaee; Sébastien Côté; Xavier Roucou; Aggregation and Amyloid Fibril Formation Induced by Chemical Dimerization of Recombinant Prion Protein in Physiological-like Conditions. Journal of Biological Chemistry 2009, 284, 30907-30916, 10.1074/jbc.m109.057950.
- Takumi Takata; Julie T. Oxford; Borries Demeler; Kirsten J. Lampi; Deamidation destabilizes and triggers aggregation of a lens protein, βA3-crystallin. Protein Science 2008, 17, 1565-1575, 10.1110/ps.035410.108.
- Yan Wei; Lan Chen; Ji Chen; Lin Ge; Rong Qiao He; Rapid glycation with D-ribose induces globular amyloid-like aggregations of BSA with high cytotoxicity to SH-SY5Y cells. BMC Cell Biology 2009, 10, 10-10, 10.1186/1471-2121-10-10.
- Lars Redecke; Stephan Binder; Mohammed I.Y. Elmallah; Rebecca Broadbent; Claudia Tilkorn; Benjamin Schulz; Patrick May; Arne Goos; Andreas Eich; Michael Rübhausen; et al. UV-light-induced conversion and aggregation of prion proteins. Free Radical Biology and Medicine 2009, 46, 1353-1361, 10.1016/j.freeradbiomed.2009.02.013.
- Shouvik Roy; Bruce D. Mason; Christian S. Schöneich; John F. Carpenter; Thomas C. Boone; Bruce A. Kerwin; Light-induced aggregation of type I soluble tumor necrosis factor receptor. Journal of Pharmaceutical Sciences 2009, 98, 3182-3199, 10.1002/jps.21750.
- Shanghao Li; Roger M. Leblanc; Aggregation of Insulin at the Interface. The Journal of Physical Chemistry B 2013, 118, 1181-1188, 10.1021/jp4101202.
- Silvia Campioni; Guillaume Carret; Sophia Jordens; Lucrèce Nicoud; Raffaele Mezzenga; Roland Riek; The Presence of an Air–Water Interface Affects Formation and Elongation of α-Synuclein Fibrils. Journal of the American Chemical Society 2014, 136, 2866-2875, 10.1021/ja412105t.
- Ben J. Trigg; Chiu Fan Lee; David J. Vaux; Létitia Jean; The air–water interface determines the outcome of seeding during amyloidogenesis. Biochemical Journal 2013, 456, 67-80, 10.1042/bj20130605.
- Létitia Jean; Chiu Fan Lee; David J. Vaux; Enrichment of Amyloidogenesis at an Air-Water Interface. Biophysical Journal 2012, 102, 1154-1162, 10.1016/j.bpj.2012.01.041.
- Létitia Jean; Chiu Fan Lee; Chongsoo Lee; Michael Shaw; David J. Vaux; Competing discrete interfacial effects are critical for amyloidogenesis. The FASEB Journal 2009, 24, 309-317, 10.1096/fj.09-137653.
- Anna Pavlova; Chi-Yuan Cheng; Maia Kinnebrew; John Lew; Frederick W. Dahlquist; Songi Han; Protein structural and surface water rearrangement constitute major events in the earliest aggregation stages of tau. Proceedings of the National Academy of Sciences 2015, 113, E127-E136, 10.1073/pnas.1504415113.
- Thibaut Frachon; Franz Bruckert; Quentin Le Masne; Emmanuel Monnin; Marianne Weidenhaupt; Insulin Aggregation at a Dynamic Solid–Liquid–Air Triple Interface. Langmuir 2016, 32, 13009-13019, 10.1021/acs.langmuir.6b03314.
- V. Sluzky; J. A. Tamada; A. M. Klibanov; R. Langer; Kinetics of insulin aggregation in aqueous solutions upon agitation in the presence of hydrophobic surfaces.. Proceedings of the National Academy of Sciences 1991, 88, 9377-9381, 10.1073/pnas.88.21.9377.
- Mark Duerkop; Eva Berger; Astrid Dürauer; Alois Jungbauer; Impact of Cavitation, High Shear Stress and Air/Liquid Interfaces on Protein Aggregation. Biotechnology Journal 2018, 13, e1800062, 10.1002/biot.201800062.
- Murali Jayaraman; Patrick M. Buck; Arun Alphonse Ignatius; Kevin R. King; Wei Wang; Agitation-induced aggregation and subvisible particulate formation in model proteins. European Journal of Pharmaceutics and Biopharmaceutics 2014, 87, 299-309, 10.1016/j.ejpb.2014.01.004.
- Sylvia Kiese; Astrid Papppenberger; Wolfgang Friess; Hanns-Christian Mahler; Shaken, Not Stirred: Mechanical Stress Testing of an IgG1 Antibody. Journal of Pharmaceutical Sciences 2008, 97, 4347-4366, 10.1002/jps.21328.
- R. Matthew Fesinmeyer; Sabine Hogan; Atul Saluja; Stephen R. Brych; Eva Kras; Linda O. Narhi; David N. Brems; Yatin R. Gokarn; Effect of Ions on Agitation- and Temperature-Induced Aggregation Reactions of Antibodies. Pharmaceutical Research 2008, 26, 903-913, 10.1007/s11095-008-9792-z.
- Jun Zhang; Elizabeth M. Topp; Protein G, Protein A and Protein A-Derived Peptides Inhibit the Agitation Induced Aggregation of IgG. Molecular Pharmaceutics 2012, 9, 622-628, 10.1021/mp200548x.
- Renuka Thirumangalathu; Sampathkumar Krishnan; Margaret Speed Ricci; David N. Brems; Theodore W. Randolph; John F. Carpenter; Silicone Oil- and Agitation-Induced Aggregation of a Monoclonal Antibody in Aqueous Solution. Journal of Pharmaceutical Sciences 2009, 98, 3167-3181, 10.1002/jps.21719.
- Lotte Krielgaard; Latoya S. Jones; Theodore W. Randolph; Sven Frokjaer; James M. Flink; Mark C. Manning; John F. Carpenter; Effect of tween 20 on freeze-thawing- and agitation-induced aggregation of recombinant human factor XIII. Journal of Pharmaceutical Sciences 1998, 87, 1597-1603, 10.1021/js980126i.
- Alireza Abdolvahabi; Yunhua Shi; Sanaz Rasouli; Corbin M. Croom; Aleksandra Chuprin; Bryan F. Shaw; How Do Gyrating Beads Accelerate Amyloid Fibrillization?. Biophysical Journal 2017, 112, 250-264, 10.1016/j.bpj.2016.12.004.
- Lisa Kueltzo; W.E.I. Wang; Theodore W. Randolph; John F. Carpenter; Effects of Solution Conditions, Processing Parameters, and Container Materials on Aggregation of a Monoclonal Antibody during Freeze-Thawing. Journal of Pharmaceutical Sciences 2008, 97, 1801-1812, 10.1002/jps.21110.
- Tatiana Perevozchikova; Hirsh Nanda; Douglas P. Nesta; Christopher J. Roberts; Protein Adsorption, Desorption, and Aggregation Mediated by Solid-Liquid Interfaces. Journal of Pharmaceutical Sciences 2015, 104, 1946-1959, 10.1002/jps.24429.
- Alana Gerhardt; Nicole R. Mcgraw; Daniel Schwartz; Jared Bee; John F. Carpenter; Theodore W. Randolph; Protein Aggregation and Particle Formation in Prefilled Glass Syringes. Journal of Pharmaceutical Sciences 2014, 103, 1601-1612, 10.1002/jps.23973.
- Pinaki Basu; Sampathkumar Sampathkumarkrishnan; Renuka Thirumangalathu; Theodore W. Randolph; John F. Carpenter; IgG1 Aggregation and Particle Formation Induced by Silicone–water Interfaces on Siliconized Borosilicate Glass Beads: A Model for Siliconized Primary Containers. Journal of Pharmaceutical Sciences 2013, 102, 852-865, 10.1002/jps.23434.
- Innocent B. Bekard; Peter Asimakis; Joseph Bertolini; Dave E. Dunstan; The effects of shear flow on protein structure and function. Biopolymers 2011, 95, 733-745, 10.1002/bip.21646.
- Enhong Cao; Yahuei Chen; Zhanfeng Cui; Peter R. Foster; Effect of freezing and thawing rates on denaturation of proteins in aqueous solutions. Biotechnology and Bioengineering 2003, 82, 684-690, 10.1002/bit.10612.
- Yifat Miller; Buyong Ma; Ruth Nussinov; Zinc ions promote Alzheimer A aggregation via population shift of polymorphic states. Proceedings of the National Academy of Sciences 2010, 107, 9490-9495, 10.1073/pnas.0913114107.
- Kevin Pagel; Tomomi Seri; Hans von Berlepsch; Jan Griebel; Reinhard Kirmse; Christoph Böttcher; Beate Koksch; How Metal Ions Affect Amyloid Formation: Cu2+- and Zn2+-Sensitive Peptides. ChemBioChem 2008, 9, 531-536, 10.1002/cbic.200700656.
- Maria Hoernke; Dr. Beate Koksch; Dr. Gerald Brezesinski; Amyloidogenic Peptides at Hydrophobic-Hydrophilic Interfaces: Coordination Affinities and the Chelate Effect Dictate the Competitive Binding of Cu2+ and Zn2+. ChemPhysChem 2011, 12, 2225-2229, 10.1002/cphc.201100215.
- M. Hoernke; B. Koksch; G. Brezesinski; Influence of the hydrophobic interface and transition metal ions on the conformation of amyloidogenic model peptides. Biophysical Chemistry 2010, 150, 64-72, 10.1016/j.bpc.2010.02.014.
- Maria Hoernke; Jessica A. Falenski; Christian Schwieger; Beate Koksch; Gerald Brezesinski; Triggers for β-Sheet Formation at the Hydrophobic–Hydrophilic Interface: High Concentration, In-Plane Orientational Order, and Metal Ion Complexation. Langmuir 2011, 27, 14218-14231, 10.1021/la203016z.
- Jing Zhang; Xiang Y. Liu; Effect of protein–protein interactions on protein aggregation kinetics. The Journal of Chemical Physics 2003, 119, 10972, 10.1063/1.1622380.
- Celine Galvagnion; Alexander K Buell; Georg Meisl; Thomas Michaels; Michele Vendruscolo; Tuomas Knowles; Christopher M Dobson; Lipid vesicles trigger α-synuclein aggregation by stimulating primary nucleation. Nature Chemical Biology 2015, 11, 229-234, 10.1038/nchembio.1750.
- Galyna Gorbenko; Valeriya M. Ioffe; Paavo K.J. Kinnunen; Binding of Lysozyme to Phospholipid Bilayers: Evidence for Protein Aggregation upon Membrane Association. Biophysical Journal 2007, 93, 140-153, 10.1529/biophysj.106.102749.
- Evelyne Terzi; Günter Hölzemann; Joachim Seelig; Self-association of β-Amyloid Peptide (1–40) in Solution and Binding to Lipid Membranes. Journal of Molecular Biology 1995, 252, 633-642, 10.1006/jmbi.1995.0525.
- Hongxia Zhao; Esa K. J. Tuominen; Paavo K. J. Kinnunen; Formation of Amyloid Fibers Triggered by Phosphatidylserine-Containing Membranes. Biochemistry 2004, 43, 10302-10307, 10.1021/bi049002c.
- Emma Sparr; Maarten Engel; Dmitri V. Sakharov; Mariette Sprong; Jet Jacobs; Ben De Kruijff; Jo W.M. Höppener; J. Antoinette Killian; Islet amyloid polypeptide-induced membrane leakage involves uptake of lipids by forming amyloid fibers. FEBS Letters 2004, 577, 117-120, 10.1016/j.febslet.2004.09.075.
- Lv, Z.; Hashemi, M.; Banerjee, S.; Zagorski, K.; Rochet, J.-C.; Lyubchenko, Y.L. Phospholipid membranes promote the early stage assembly of α-synuclein aggregates. bioRxiv 2018, 295782.
- Abha Chauhan; Indrani Ray; Ved P. S. Chauhan; Interaction of amyloid beta-protein with anionic phospholipids: possible involvement of Lys28 and C-terminus aliphatic amino acids.. Neurochemical Research 2000, 25, 423-429, 10.1023/a:1007509608440.
- Canay Ege; Ka Yee C. Lee; Insertion of Alzheimer’s Aβ40 Peptide into Lipid Monolayers. Biophysical Journal 2004, 87, 1732-1740, 10.1529/biophysj.104.043265.
- Frank Ferrone; [17] Analysis of protein aggregation kinetics. Methods in Enzymology 1999, 309, 256-274, 10.1016/s0076-6879(99)09019-9.
- Aimee M. Morris; Murielle A. Watzky; Richard G. Finke; Protein aggregation kinetics, mechanism, and curve-fitting: A review of the literature. Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics 2009, 1794, 375-397, 10.1016/j.bbapap.2008.10.016.
- Paolo Arosio; Tuomas P. J. Knowles; Sara Linse; On the lag phase in amyloid fibril formation. Physical Chemistry Chemical Physics 2015, 17, 7606-7618, 10.1039/c4cp05563b.
- James D. Harper; Peter T. Lansbury; MODELS OF AMYLOID SEEDING IN ALZHEIMER'S DISEASE AND SCRAPIE: Mechanistic Truths and Physiological Consequences of the Time-Dependent Solubility of Amyloid Proteins. Annual Review of Biochemistry 1997, 66, 385-407, 10.1146/annurev.biochem.66.1.385.
- Brian O'Nuallain; Shankaramma Shivaprasad; Indu Kheterpal; Ronald Wetzel; Thermodynamics of Aβ(1−40) Amyloid Fibril Elongation. Biochemistry 2005, 44, 12709-12718, 10.1021/bi050927h.
- Murielle A. Watzky; Aimee M. Morris; Eric D. Ross; Richard G. Finke; Fitting Yeast and Mammalian Prion Aggregation Kinetic Data with the Finke−Watzky Two-Step Model of Nucleation and Autocatalytic Growth†. Biochemistry 2008, 47, 10790-10800, 10.1021/bi800726m.
- ‡ Aimee M. Morris; Murielle Watzky; § And Jeffrey N. Agar; ‡ Richard G. Finke; Fitting Neurological Protein Aggregation Kinetic Data via a 2-Step, Minimal/“Ockham's Razor” Model: The Finke−Watzky Mechanism of Nucleation Followed by Autocatalytic Surface Growth. Biochemistry 2008, 47, 2413-2427, 10.1021/bi701899y.
- Murielle A. Watzky; Richard G. Finke; Transition Metal Nanocluster Formation Kinetic and Mechanistic Studies. A New Mechanism When Hydrogen Is the Reductant: Slow, Continuous Nucleation and Fast Autocatalytic Surface Growth. Journal of the American Chemical Society 1997, 119, 10382-10400, 10.1021/ja9705102.
- Bertrand Morel; Maria Paz Carrasco; Samuel Jurado; Carmen Marco; Francisco Conejero-Lara; Dynamic micellar oligomers of amyloid beta peptides play a crucial role in their aggregation mechanisms. Physical Chemistry Chemical Physics 2018, 20, 20597-20614, 10.1039/c8cp02685h.
- Jeremy D. Schmit; Kingshuk Ghosh; Ken Dill; What Drives Amyloid Molecules To Assemble into Oligomers and Fibrils?. Biophysical Journal 2011, 100, 450-458, 10.1016/j.bpj.2010.11.041.
- Filip Hasecke; Tatiana Miti; Carlos Perez; Jeremy Barton; Daniel Schölzel; Lothar Gremer; Clara S. R. Grüning; Garrett Matthews; Georg Meisl; Tuomas P. J. Knowles; et al. Origin of metastable oligomers and their effects on amyloid fibril self-assembly. Chemical Science 2018, 9, 5937-5948, 10.1039/c8sc01479e.
- Evan Powers; David L. Powers; The Kinetics of Nucleated Polymerizations at High Concentrations: Amyloid Fibril Formation Near and Above the “Supercritical Concentration”. Biophysical Journal 2006, 91, 122-132, 10.1529/biophysj.105.073767.
- Tricia R. Serio; Anil G. Cashikar; Anthony S. Kowal; George J. Sawicki; Jahan J. Moslehi; Louise Serpell; Morton F. Arnsdorf; Susan L. Lindquist; Nucleated Conformational Conversion and the Replication of Conformational Information by a Prion Determinant. Science 2000, 289, 1317-1321, 10.1126/science.289.5483.1317.
- Ziao Fu; Darryl Aucoin; Judianne Davis; William E. Van Nostrand; Steven O. Smith; Mechanism of Nucleated Conformational Conversion of Aβ42. Biochemistry 2015, 54, 4197-4207, 10.1021/acs.biochem.5b00467.
- Jiyong Lee; Elizabeth K. Culyba; Evan Powers; Jeffery W. Kelly; Amyloid-β forms fibrils by nucleated conformational conversion of oligomers. Nature Chemical Biology 2011, 7, 602-609, 10.1038/nchembio.624.
- Sandra Chimon; Medhat A Shaibat; Christopher R Jones; Diana C Calero; Buzulagu Aizezi; Yoshitaka Ishii; Evidence of fibril-like β-sheet structures in a neurotoxic amyloid intermediate of Alzheimer's β-amyloid. Nature Structural & Molecular Biology 2007, 14, 1157-1164, 10.1038/nsmb1345.
- David Ruzafa; Bertrand Morel; Lorena Varela; Ana I. Azuaga; Francisco Conejero-Lara; Characterization of Oligomers of Heterogeneous Size as Precursors of Amyloid Fibril Nucleation of an SH3 Domain: An Experimental Kinetics Study. PLOS ONE 2012, 7, e49690, 10.1371/journal.pone.0049690.
- David Ruzafa; Francisco Conejero-Lara; Bertrand Morel; Modulation of the stability of amyloidogenic precursors by anion binding strongly influences the rate of amyloid nucleation. Physical Chemistry Chemical Physics 2013, 15, 15508-15517, 10.1039/c3cp52313f.
- Bertrand Morel; David Ruzafa; Francisco Conejero-Lara; SH3 Domains as Suitable Models to Study Amyloid Aggregation. SH Domains 2015, 15(37), 1-15, 10.1007/978-3-319-20098-9_1.
- Nicolas Fay; Yuji Inoue; Luc Bousset; Hideki Taguchi; Ronald Melki; Assembly of the Yeast Prion Ure2p into Protein Fibrils. Journal of Biological Chemistry 2003, 278, 30199-30205, 10.1074/jbc.m303000200.
- A. M. Bhattacharyya; A. K. Thakur; R. Wetzel; Polyglutamine aggregation nucleation: Thermodynamics of a highly unfavorable protein folding reaction. Proceedings of the National Academy of Sciences 2005, 102, 15400-15405, 10.1073/pnas.0501651102.
- Avanish S. Parmar; Paul E. Gottschall; Martin Muschol; Pre-assembled clusters distort crystal nucleation kinetics in supersaturated lysozyme solutions. Biophysical Chemistry 2007, 129, 224-234, 10.1016/j.bpc.2007.06.002.
- Shannon E. Hill; Joshua Robinson; Garrett Matthews; Martin Muschol; Amyloid Protofibrils of Lysozyme Nucleate and Grow Via Oligomer Fusion. Biophysical Journal 2009, 96, 3781-3790, 10.1016/j.bpj.2009.01.044.
- Joseph T. Jarrett; Peter T. Lansbury; Seeding “one-dimensional crystallization” of amyloid: A pathogenic mechanism in Alzheimer's disease and scrapie?. Cell 1993, 73, 1055-1058, 10.1016/0092-8674(93)90635-4.
- S. I. A. Cohen; S. Linse; L. M. Luheshi; E. Hellstrand; D. A. White; L. Rajah; Daniel Otzen; Michele Vendruscolo; C. M. Dobson; T. P. J. Knowles; et al. Proliferation of amyloid- 42 aggregates occurs through a secondary nucleation mechanism. Proceedings of the National Academy of Sciences 2013, 110, 9758-9763, 10.1073/pnas.1218402110.
- Georg Meisl; Xiaoting Yang; Christopher M. Dobson; Sara Linse; Tuomas P. J. Knowles; Modulation of electrostatic interactions to reveal a reaction network unifying the aggregation behaviour of the Aβ42 peptide and its variants. Chemical Science 2017, 8, 4352-4362, 10.1039/c7sc00215g.
- Samuel I. A. Cohen; Michele Vendruscolo; Mark E. Welland; Christopher M. Dobson; Eugene M. Terentjev; Tuomas P. J. Knowles; Nucleated polymerization with secondary pathways. I. Time evolution of the principal moments. The Journal of Chemical Physics 2011, 135, 065105-065105, 10.1063/1.3608916.
- Samuel I. A. Cohen; Michele Vendruscolo; Christopher M. Dobson; Tuomas P. J. Knowles; Nucleated polymerization with secondary pathways. II. Determination of self-consistent solutions to growth processes described by non-linear master equations. The Journal of Chemical Physics 2011, 135, 065106-065106, 10.1063/1.3608917.
- Tuomas P. J. Knowles; Christopher A. Waudby; Glyn L. Devlin; Samuel I. A. Cohen; Adriano Aguzzi; Michele Vendruscolo; Eugene M. Terentjev; Mark E. Welland; Christopher M. Dobson; An Analytical Solution to the Kinetics of Breakable Filament Assembly. Science 2009, 326, 1533-1537, 10.1126/science.1178250.
- Sara Linse; Monomer-dependent secondary nucleation in amyloid formation. Biophysical Reviews 2017, 9, 329-338, 10.1007/s12551-017-0289-z.
- Mattias Törnquist; Thomas C. T. Michaels; Kalyani Sanagavarapu; Xiaoting Yang; Georg Meisl; Samuel I. A. Cohen; Tuomas P. J. Knowles; Sara Linse; Secondary nucleation in amyloid formation. Chemical Communications 2018, 54, 8667-8684, 10.1039/c8cc02204f.
- Georg Meisl; Xiaoting Yang; Erik Hellstrand; Birgitta Frohm; Julius Kirkegaard; Samuel I. A. Cohen; Christopher M. Dobson; Sara Linse; Tuomas Knowles; Differences in nucleation behavior underlie the contrasting aggregation kinetics of the Aβ40 and Aβ42 peptides. Proceedings of the National Academy of Sciences 2014, 111, 9384-9389, 10.1073/pnas.1401564111.
- Gayathri Ramachandran; Jayant B. Udgaonkar; Evidence for the Existence of a Secondary Pathway for Fibril Growth during the Aggregation of Tau. Journal of Molecular Biology 2012, 421, 296-314, 10.1016/j.jmb.2012.01.007.
- Alexander K. Buell; Celine Galvagnion; Ricardo Gaspar; Emma Sparr; Michele Vendruscolo; Tuomas Knowles; Sara Linse; Christopher M. Dobson; Solution conditions determine the relative importance of nucleation and growth processes in -synuclein aggregation. Proceedings of the National Academy of Sciences 2014, 111, 7671-7676, 10.1073/pnas.1315346111.
- Ricardo Gaspar; Georg Meisl; Alexander K. Buell; Laurence Young; Clemens F. Kaminski; Tuomas P. J. Knowles; Emma Sparr; Sara Linse; Secondary nucleation of monomers on fibril surface dominatesα-synuclein aggregation and provides autocatalytic amyloid amplification. Quarterly Reviews of Biophysics 2017, 50, e6-e6, 10.1017/s0033583516000172.
- Shae Padrick; Andrew D. Miranker; Islet Amyloid: Phase Partitioning and Secondary Nucleation Are Central to the Mechanism of Fibrillogenesis. Biochemistry 2002, 41, 4694-4703, 10.1021/bi0160462.
- Vito Foderà; Fabio Librizzi; Minna Groenning; Marco van de Weert; Maurizio Leone; Secondary Nucleation and Accessible Surface in Insulin Amyloid Fibril Formation. The Journal of Physical Chemistry B 2008, 112, 3853-3858, 10.1021/jp710131u.
- Dushyant Kumar Garg; Bishwajit Kundu; Clues for divergent, polymorphic amyloidogenesis through dissection of amyloid forming steps of bovine carbonic anhydrase and its critical amyloid forming stretch. Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics 2016, 1864, 794-804, 10.1016/j.bbapap.2016.03.019.
- Samuel I. A. Cohen; Michele Vendruscolo; Christopher M. Dobson; Tuomas P. J. Knowles; Nucleated Polymerisation in the Presence of Pre-Formed Seed Filaments. International Journal of Molecular Sciences 2011, 12, 5844-5852, 10.3390/ijms12095844.
- Rodrigo Morales; Ines Moreno-Gonzalez; Claudio Soto; Cross-Seeding of Misfolded Proteins: Implications for Etiology and Pathogenesis of Protein Misfolding Diseases. PLOS Pathogens 2013, 9, e1003537, 10.1371/journal.ppat.1003537.
- Lary C. Walker; Marc I. Diamond; Karen E. Duff; Bradley T. Hyman; Mechanisms of Protein Seeding in Neurodegenerative Diseases. JAMA Neurology 2013, 70, 304-310, 10.1001/jamaneurol.2013.1453.
- J. H. Come; P. E. Fraser; P. T. Lansbury; A kinetic model for amyloid formation in the prion diseases: importance of seeding.. Proceedings of the National Academy of Sciences 1993, 90, 5959-5963, 10.1073/pnas.90.13.5959.
- Patrik Brundin; Ronald Melki; Ron R Kopito; Prion-like transmission of protein aggregates in neurodegenerative diseases. Nature Reviews Molecular Cell Biology 2010, 11, 301-307, 10.1038/nrm2873.
- Lary C. Walker; Mathias Jucker; Neurodegenerative Diseases: Expanding the Prion Concept. Annual Review of Neuroscience 2015, 38, 87-103, 10.1146/annurev-neuro-071714-033828.
- Mathias Jucker; Lary C. Walker; Pathogenic protein seeding in alzheimer disease and other neurodegenerative disorders. Annals of Neurology 2011, 70, 532-540, 10.1002/ana.22615.
- Kenjiro Ono; Ryoichi Takahashi; Tokuhei Ikeda; Masahito Yamada; Cross-seeding effects of amyloid β-protein and α-synuclein. Journal of Neurochemistry 2012, 122, 883-890, 10.1111/j.1471-4159.2012.07847.x.
- Fumio Oosawa; Michiki Kasai; A theory of linear and helical aggregations of macromolecules. Journal of Molecular Biology 1962, 4, 10-21, 10.1016/s0022-2836(62)80112-0.
- Oosawa, F.; Asakura, S. Thermodynamics of the Polymerization of Protein; Academic Press: London, UK, 1975.
- David Ruzafa; Lorena Varela; Ana I. Azuaga; Francisco Conejero-Lara; Bertrand Morel; Mapping the structure of amyloid nucleation precursors by protein engineering kinetic analysis. Physical Chemistry Chemical Physics 2013, 16, 2989-3000, 10.1039/c3cp54383h.
- Yukiko Hori; Tadafumi Hashimoto; Yosuke Wakutani; Katsuya Urakami; Kenji Nakashima; Margaret M. Condron; Satoshi Tsubuki; Takaomi C. Saido; David B. Teplow; Takeshi Iwatsubo; et al. The Tottori (D7N) and English (H6R) Familial Alzheimer Disease Mutations Accelerate Aβ Fibril Formation without Increasing Protofibril Formation. Journal of Biological Chemistry 2007, 282, 4916-4923, 10.1074/jbc.m608220200.
- Santosh Kumar; Jayant B. Udgaonkar; Conformational Conversion May Precede or Follow Aggregate Elongation on Alternative Pathways of Amyloid Protofibril Formation. Journal of Molecular Biology 2009, 385, 1266-1276, 10.1016/j.jmb.2008.11.033.
- Amy R. Hurshman; Joleen T. White; Evan T. Powers; Jeffery W. Kelly; Transthyretin Aggregation under Partially Denaturing Conditions Is a Downhill Polymerization. Biochemistry 2004, 43, 7365-7381, 10.1021/bi049621l.
- Tiago Q. Faria; Zaida L. Almeida; Pedro F. Cruz; Catarina S. H. Jesus; Pedro Castanheira; Rui M. M. Brito; A look into amyloid formation by transthyretin: aggregation pathway and a novel kinetic model. Physical Chemistry Chemical Physics 2015, 17, 7255-7263, 10.1039/c4cp04549a.
- Brian O'nuallain; Darragh B. Freir; Andrew J. Nicoll; Emmanuel Risse; Neil Ferguson; Caroline E. Herron; John Collinge; Dominic M. Walsh; Amyloid -Protein Dimers Rapidly Form Stable Synaptotoxic Protofibrils. The Journal of Neuroscience 2010, 30, 14411-14419, 10.1523/jneurosci.3537-10.2010.
- Vijayaraghavan Rangachari; Brenda D. Moore; Dana Kim Reed; Leilani K. Sonoda; Alexander W. Bridges; Erin Conboy; David Hartigan; Terrone L. Rosenberry; Amyloid-β(1−42) Rapidly Forms Protofibrils and Oligomers by Distinct Pathways in Low Concentrations of Sodium Dodecylsulfate. Biochemistry 2007, 46, 12451-12462, 10.1021/bi701213s.
- Gayathri Ramachandran; Jayant B. Udgaonkar; Understanding the Kinetic Roles of the Inducer Heparin and of Rod-like Protofibrils during Amyloid Fibril Formation by Tau Protein. Journal of Biological Chemistry 2011, 286, 38948-38959, 10.1074/jbc.m111.271874.
- Josué Juárez; Pablo Taboada; Victor Mosquera; Existence of Different Structural Intermediates on the Fibrillation Pathway of Human Serum Albumin. Biophysical Journal 2009, 96, 2353-2370, 10.1016/j.bpj.2008.12.3901.
- Nikolaj K. Holm; Stine K. Jespersen; Lise V. Thomassen; Tine Y. Wolff; Pankaj Sehgal; Line A. Thomsen; Gunna Christiansen; Christian Beyschau Andersen; Anders D. Knudsen; Daniel Otzen; et al. Aggregation and fibrillation of bovine serum albumin. Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics 2007, 1774, 1128-1138, 10.1016/j.bbapap.2007.06.008.
- Silvia Campioni; Maria Francesca Mossuto; Silvia Torrassa; Giulia Calloni; Patrizia Polverino de Laureto; Annalisa Relini; Angelo Fontana; Fabrizio Chiti; Conformational properties of the aggregation precursor state of HypF-N. Journal of Molecular Biology 2008, 379, 554-567, 10.1016/j.jmb.2008.04.002.
- Patrizia Marinelli; Susanna Navarro; Manuel Bano Polo; Bertrand Morel; Ricardo Graña-Montes; Anna Sabé; Francesc Canals; Maria Rosario Fernandez; Francisco Conejero Lara; Salvador Ventura; et al. Global Protein Stabilization Does Not Suffice to Prevent Amyloid Fibril Formation. ACS Chemical Biology 2018, 13, 2094-2105, 10.1021/acschembio.8b00607.
- Martino Calamai; Niccolo Taddei; Massimo Stefani; Giampietro Ramponi; Fabrizio Chiti; Relative Influence of Hydrophobicity and Net Charge in the Aggregation of Two Homologous Proteins. Biochemistry 2003, 42, 15078-15083, 10.1021/bi030135s.
- Shuo Yang; Michael D.W. Griffin; Katrina J. Binger; Peter Schuck; Geoffrey J. Howlett; An Equilibrium Model for Linear and Closed-Loop Amyloid Fibril Formation. Journal of Molecular Biology 2012, 421, 364-377, 10.1016/j.jmb.2012.02.026.
- Michael D.W. Griffin; Melva L.Y. Mok; LeAnne M. Wilson; Chi L.L. Pham; Lynne J. Waddington; Matthew A. Perugini; Geoffrey J. Howlett; Phospholipid Interaction Induces Molecular-level Polymorphism in Apolipoprotein C-II Amyloid Fibrils via Alternative Assembly Pathways. Journal of Molecular Biology 2008, 375, 240-256, 10.1016/j.jmb.2007.10.038.
- Bertrand Morel; Lorena Varela; Ana I. Azuaga; Francisco Conejero-Lara; Environmental Conditions Affect the Kinetics of Nucleation of Amyloid Fibrils and Determine Their Morphology. Biophysical Journal 2010, 99, 3801-3810, 10.1016/j.bpj.2010.10.039.
- Jesús Zurdo; J. Iñaki Guijarro; Jose L Jiménez; Helen R Saibil; Christopher M Dobson; Dependence on solution conditions of aggregation and amyloid formation by an SH3 domain. Journal of Molecular Biology 2001, 311, 325-340, 10.1006/jmbi.2001.4858.
- Lorena Varela; Bertrand Morel; Ana I. Azuaga; Francisco Conejero-Lara; A single mutation in an SH3 domain increases amyloid aggregation by accelerating nucleation, but not by destabilizing thermodynamically the native state. FEBS Letters 2009, 583, 801-806, 10.1016/j.febslet.2009.01.033.
- Alexander L. Watters; Pritilekha Deka; Colin Corrent; David Callender; Gabriele Varani; Tobin Sosnick; David Baker; The Highly Cooperative Folding of Small Naturally Occurring Proteins Is Likely the Result of Natural Selection. Cell 2007, 128, 613-624, 10.1016/j.cell.2006.12.042.
- Michele Vendruscolo; E. Paci; M. Karplus; C. M. Dobson; Structures and relative free energies of partially folded states of proteins. Proceedings of the National Academy of Sciences 2003, 100, 14817-14821, 10.1073/pnas.2036516100.
- David Brockwell; Sheena E Radford; Intermediates: ubiquitous species on folding energy landscapes?. Current Opinion in Structural Biology 2007, 17, 30-37, 10.1016/j.sbi.2007.01.003.
- Thomas Jahn; Sheena E. Radford; Folding versus aggregation: Polypeptide conformations on competing pathways. Archives of Biochemistry and Biophysics 2008, 469, 100-117, 10.1016/j.abb.2007.05.015.
- Konstantin K. Turoverov; Irina M. Kuznetsova; Vladimir N. Uversky; The protein kingdom extended: Ordered and intrinsically disordered proteins, their folding, supramolecular complex formation, and aggregation. Progress in Biophysics and Molecular Biology 2010, 102, 73-84, 10.1016/j.pbiomolbio.2010.01.003.
- Yoshiki Sekijima; Luke Wiseman; Jeanne Matteson; Per Hammarstrom; Sean R. Miller; Anu R. Sawkar; William E. Balch; Jeffery W. Kelly; The Biological and Chemical Basis for Tissue-Selective Amyloid Disease. Cell 2005, 121, 73-85, 10.1016/j.cell.2005.01.018.
- Samuel I.A. Cohen; Michele Vendruscolo; Christopher M. Dobson; Tuomas Knowles; From Macroscopic Measurements to Microscopic Mechanisms of Protein Aggregation. Journal of Molecular Biology 2012, 421, 160-171, 10.1016/j.jmb.2012.02.031.
- Katy E. Routledge; Gian Gaetano Tartaglia; Geoffrey W. Platt; Michele Vendruscolo; Sheena E. Radford; Competition between Intramolecular and Intermolecular Interactions in an Amyloid-Forming Protein. Journal of Molecular Biology 2009, 389, 776-786, 10.1016/j.jmb.2009.04.042.
- A.J Modler; K Gast; G Lutsch; G Damaschun; Assembly of Amyloid Protofibrils via Critical Oligomers—A Novel Pathway of Amyloid Formation. Journal of Molecular Biology 2003, 325, 135-148, 10.1016/s0022-2836(02)01175-0.
- D. Thirumalai; G B Reddy; Are native proteins metastable?. Nature Chemistry 2011, 3, 910-911, 10.1038/nchem.1207.
- Fabrizio Chiti; Niccolò Taddei; Fabiana Baroni; Cristina Capanni; Massimo Stefani; Giampietro Ramponi; Christopher M. Dobson; Kinetic partitioning of protein folding and aggregation. Nature Genetics 2002, 9, 137-143, 10.1038/nsb752.
- José L. Jiménez; Ewan J. Nettleton; Mario Bouchard; Carol Robinson; Christopher M. Dobson; Helen R. Saibil; The protofilament structure of insulin amyloid fibrils. Proceedings of the National Academy of Sciences 2002, 99, 9196-9201, 10.1073/pnas.142459399.
- Timo Eichner; Sheena Radford; A Diversity of Assembly Mechanisms of a Generic Amyloid Fold. Molecular Cell 2011, 43, 8-18, 10.1016/j.molcel.2011.05.012.
- Andrew J. Baldwin; Tuomas P. J. Knowles; Gian Gaetano Tartaglia; Anthony W. Fitzpatrick; Glyn L. Devlin; Sarah Lucy Shammas; Christopher A. Waudby; Maria F. Mossuto; Sarah Meehan; Sally L. Gras; et al. Metastability of Native Proteins and the Phenomenon of Amyloid Formation. Journal of the American Chemical Society 2011, 133, 14160-14163, 10.1021/ja2017703.
- Song-Ho Chong; Sihyun Ham; Distinct Role of Hydration Water in Protein Misfolding and Aggregation Revealed by Fluctuating Thermodynamics Analysis. Accounts of Chemical Research 2015, 48, 956-965, 10.1021/acs.accounts.5b00032.
- Margaret Sunde; Colin C. F. Blake; From the globular to the fibrous state: protein structure and structural conversion in amyloid formation. Quarterly Reviews of Biophysics 1998, 31, 1-39, 10.1017/s0033583598003400.
- Stephanie M Dorta-Estremera; Jingjing Li; Wei Cao; Rapid Generation of Amyloid from Native Proteins In vitro. Journal of Visualized Experiments 2013, 82, e50869-e50869, 10.3791/50869.
- Lukasz Goldschmidt; Poh K. Teng; Roland Riek; David Eisenberg; Identifying the amylome, proteins capable of forming amyloid-like fibrils. Proceedings of the National Academy of Sciences 2010, 107, 3487-3492, 10.1073/pnas.0915166107.
- Ronald Wetzel; Kinetics and Thermodynamics of Amyloid Fibril Assembly. Accounts of Chemical Research 2006, 39, 671-679, 10.1021/ar050069h.
