Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Oncology
Tumour hypoxia is significantly correlated with patient survival and treatment outcomes. At the molecular level, hypoxia is a major driving factor for tumour progression and aggressiveness. There have been extensive studies to target tumour hypoxia and here are some examples of historical methods as well as new approaches.
- hypoxia
- cancer
- tirapazamine
- hyperthermia
- carbogen breathing
- tumour metabolism
1. Introduction
The presence of low oxygen concentrations, known as hypoxia, is a prevalent characteristic of the microenvironment of solid tumours [1]. Tumour hypoxia causes aggressive tumour progression and treatment resistance, which leads to poor patient survival. Hypoxia arises from an imbalance between oxygen consumption and delivery, which is caused by rapid tumour cell proliferation and an inefficient blood supply carrying oxygen and nutrients [2]. In radiobiology, tumour hypoxia, which is defined as an oxygen tension below 10 mmHg, is significantly correlated with radiation resistance due to the role of oxygen in the ”fixation” of DNA damage. In addition to direct DNA damage by single- or double-strand breaks (SSB or DSB), the ionizing radiation causes indirect DNA damage by producing free radicals that reinforce the damage and make it permanent. However, the reduced production of free radicals under hypoxic conditions allows for the repair of the radiation-induced damage before it is “fixed” [3]. A summary of the approaches discussed here is depicted in Figure 1.
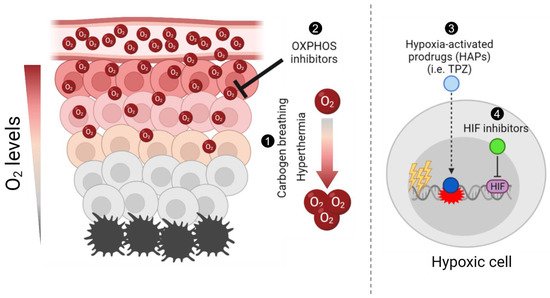
Figure 1. Schematic summary of different approaches to target tumour hypoxia. Briefly, (1) carbogen breathing and hyperthermia aim to increase the overall oxygen concentration in tumours, enhancing radiosensitisation; (2) Oxidative phosphorylation (OXPHOS) inhibitors target mitochondrial metabolism in well-oxygenated cells to promote an increase and diffusion of the overall oxygen into hypoxic regions and enhance radiation-induced damage; (3) hypoxia-activated prodrugs (HAPs), such as tirapazamine (TPZ), are reduced into their active cytotoxic form in hypoxic cells; and (4) the hypoxia-inducible factor (HIF) inhibitors aim to inhibit the hypoxia-induced activation of HIF, hence selectively targeting hypoxic cells. The figure was generated using Biorender.com (Accessed on 21 October 2021).
2. Carbogen Breathing
The most relevant approach to overcome tumour hypoxia is to promote oxygen delivery. Hyperbaric oxygen (HBO) treatment consists of breathing 100% oxygen at 2–4 times the normal atmospheric pressure, which results in saturated haemoglobin and increased oxygen in the circulation [5,6,7]. While HBO is applied during or briefly before the radiation treatment, multiple clinical studies, including multi-centre randomised trials by the medical research council (MRC) in late 1970s and mid-1980s, showed that HBO treatment improved local control in patients with head and neck squamous cell carcinoma (HNSCC) or cervical cancer, compared to normal air. However, technical challenges derived from the decompression of the hyperbaric chamber, due to the radiation equipment and severe tissue damage, limited the use of HBO in radiation treatment [8]. Thus, carbogen breathing (95% oxygen + 5% carbon dioxide) was suggested as an alternative to HBO as it could promote oxygen delivery due to vasodilation and increased blood flow induced by carbon dioxide [9,10]. Nonetheless, carbogen breathing showed mixed results, which could be attributed to inconsistencies in the amounts of time patients were subjected to carbogen breathing [11,12]. Although carbogen treatment was well-tolerated by paediatric patients with high-risk brain stem glioma, no efficacy was found when combined with radiation [9].
Nicotinamide is a vitamin B3-derived molecule and its initial radiosensitising effect was thought to be mediated by the inhibition of the DNA damage repair (DDR) pathway specifically by blocking poly(ADP-ribose) polymerase 1 (PARP1), which is responsible for nucleotide excision repair (BER) [10]. The combination of carbogen with nicotinamide seems to target both acute and chronic hypoxia. Further studies suggested that nicotinamide could reduce acute hypoxia by inhibiting intermittent vascular shut down [11,12]. Accelerated radiotherapy with carbogen and nicotinamide (ARCON) trials showed a better patient survival and outcome in bladder and laryngeal cancer [13,14,15,16]. Although ARCON trials indicated the effectiveness of carbogen breathing as an adjuvant treatment regimen, it is necessary to consider other studies questioning the use of carbon dioxide in patients and the effectiveness of carbogen or the combination of carbogen and nicotinamide when compared to HBO [17,18].
3. Hyperthermia
Hyperthermia (HT) is a treatment based on applying heat (40 to 45°C) to tissues to above physiological temperatures. HT can be directly applied to treat tumours, but due to the technical difficulties in achieving cytotoxic temperatures, it is mainly used as an adjuvant therapy in combination with radiation or chemotherapy [19,20,21,22,23,24]. HT promotes protein denaturation and increases membrane fluidity and cell cycle inhibition. In addition to its direct action against tumour cells, HT is reported to inhibit DDR and promote tumour oxygenation, which might be more clinically relevant as they are directly related to tumour radiosensitivity [25,26].
Although HT has been used since ancient times to treat a variety of diseases. Its clinical use for cancer patients was introduced in the 1900s, which was accelerated by the development of the devices to heat tumours [27,28]. In patients, HT is externally or interstitially given using microwaves, radiofrequencies, electromagnetic radiation, or ultrasound. The accumulation of evidence supporting the beneficial effects from HT in cells, animals and patients eventually led to clinical trials in combination with radiation in the 1970s and 1980s. However, mixed patient outcomes put into question the efficacy of the combination of HT and radiation. Nevertheless, the analysis of five UK-led randomised trials of radiation and HT by Vernon et al., multi-centre trials by Overgaard et al., and studies by Jones et al. and van der Zee et al. suggested that the combination of HT and radiation was beneficial for some groups of patients, including locally recurrent breast cancer, cervical cancer, and melanoma [29,30,31,32,33,34,35,36,37,38,39]. Moreover, multiple studies further supported the beneficial effects of HT in paediatric and adolescent patients with a variety of tumour types, including malignant germ cell tumours, soft tissue sarcoma, and chondrosarcomas [40,41].
The biggest clinical challenge when delivering HT is to achieve cytotoxic temperatures (>43 °C) and to overcome thermotolerance [42,43]. It is hard to apply sufficient heat to induce cell death, without causing pain and damage to normal tissues. In addition, the isothermal (homogeneous) heating of tumour tissues is a major technical limitation in trying to reach the target temperature for the treatment. Non-homogeneous tissue heating also results in the differential heat resistance of tumour areas, which is caused by the formation of heat shock proteins (HSPs) [42]. Recent technical advances enabled real-time, MRI-guided thermotherapy (or MR thermometry) to define the heated region while monitoring the temperature rise [44,45]. The meta-analyses from breast cancer, cervical cancer, and HNSCC trials still support the advantage of HT with multimodal therapy [46,47,48]. Therefore, it is still necessary to fully understand the biological effects of HT and to set up a better strategy for the treatment schedule, patient groups, and the combination of other therapeutic modalities.
Taken together, mild HT has a strong potential as an adjuvant treatment to overcome tumour hypoxia. To obtain clearer insights into the relationship between HT treatment and tumour oxygenation at the molecular level, further investigation is required. Furthermore, the underlying mechanisms of heat resistance (heat shock proteins, HIF pathways, ROS, and endothelial-to-mesenchymal transition) need to be considered to identify therapeutic targets or to better stratify patient groups into benefitting from HT [60,71,72,73,74].
4. Hypoxia-Activated Prodrugs
In the 1980s, several studies proposed targeting hypoxia by developing hypoxia-specific cytotoxins, which are now known as hypoxia-activated prodrugs (HAPs) [75,76]. HAPs are compounds that can be selectively reduced by endogenous cellular oxidoreductases to yield cytotoxic agents under hypoxic conditions. This bioreductive process is normally inhibited by molecular oxygen, which directly competes for the single electron of the initially reduced prodrug, preventing the complete reduction of the compound into its active form. The superoxide product is then detoxified by superoxide dismutase (SOD) resulting in a low toxicity in normal tissues. However, under hypoxia, the prodrug is reduced by completely yielding the active compound, thus allowing for the specific target of hypoxic regions [77].
Since the 1960s, the discovery of a direct relation between the electron affinity of the sensitising agent and the degree of radiosensitisation led to the study of nitroaromatic compounds as potential hypoxia modifiers [78]. These types of HAPs present high electron affinities, which allow them to fix or stabilise radiation-induced DNA damage making it unrepairable, and thus lethal [79]. The chemical properties of nitroaromatic drugs under hypoxia resemble those of oxygen, hence, they have often been referred as “oxygen mimetics”.
The first nitroaromatic drugs, which were tested as radiosensitisers, were metronidazole and misonidazole. These nitroaromatic molecules showed a high effectiveness both in vitro and in preclinical models [80,81]. However, clinical trials failed for both compounds, showing no increases in patient survival between the combination treatment with radiation and radiation alone [82,83,84]. The failure of these trials was attributed to a high toxicity, which was caused by a high volume of distribution and a longer half-life of the drug in humans compared to murine models. As a result, the high toxicity of these compounds limited the maximal tolerable dose, which resulted in a low radiosensitisation. To increase the efficacy of the treatment, three new nitroaromatic compounds with improved pharmacokinetic properties and reduced toxicity were developed and tested clinically: etanidazole, pimonidazole, and nimorazole. Although etanidazole was involved in phase II and phase III clinical trials, it did not show any improvement for patients with neither HNSCC nor small-cell lung cancer (SCLC) [85,86]. Pimonidazole also failed to provide evidence of the benefit of a combination treatment with radiation, when compared to only radiation in cervical carcinoma patients [87]. However, currently, pimonidazole is widely used for measuring tumour hypoxia by immunohistochemical staining [88].
In contrast to etanidazole and pimonidazole, nimorazole showed promising clinical results. It improved the radiotherapeutic effect in supraglottic and pharynx tumours without side effects in a Danish head and neck cancer study (DAHANCA) [89]. As a result, nimorazole has now become a standard treatment for HNSCC in Denmark, although it is still necessary to assess whether nimorazole directly changes the oxygenation status of patient tumours. Recently, a phase I/II clinical trial for p16-negative HNSCC reported positive results for the combination of nimorazole and radiotherapy [90]. In addition to two ongoing clinical trials for nimorazole in Denmark (NCT02661152 and NCT02976051), there is a randomised clinical trial in the UK for patients with HNSCC undergoing radiotherapy who are not suitable for synchronous chemotherapy or cetuximab (NCT01950689).
5. Tirapazamine
Following the development of the initial set of oxygen-mimetic HAPs, new agents targeting both hypoxia and DNA were developed. Several compounds have been reported to reduce tumour hypoxia in vitro and were studied in the clinic, including mitomycin C, PR-104, evofosfamide (TH-302), A4QN, and tirapazamine (TPZ), as extensively reviewed elsewhere [91,92]. In this review, we specifically focus on TPZ as its usage to target tumour hypoxia has recently been revisited and is currently being clinically tested.
TPZ or 3-amino-1,2,4-benzotriazine1,4-dioxide is a HAP which can be reduced to its cytotoxic form by one electron reductases (i.e., NADPH-dependent cytochrome P450 reductase). This type of reduction leads to the formation of a TPZ radical and the release of hydroxide radical (OH·), then the TPZ radical is further reduced to TPZ 1-oxide by acquiring another electron [93,94]. TPZ can also be reduced by two electron reductases such as NAD(P)H dehydrogenase [quinone] 1 (NQO1) to TPZ 1-oxide directly [95]. The cytotoxicity of TPZ increases many fold under hypoxia. However, both forms of the reduced TPZ induce DNA damage through SSB and DSB, therefore TPZ can also be toxic under normoxia. The cytotoxic action of TPZ under normoxia is probably linked to the production of superoxides (O2−) and the activity of NQO1 [95]. NQO1 can reduce TPZ under normoxia, TPZ-1-oxide is then oxidized back to TPZ which produces O2−.; O2− can then be converted into hydrogen peroxide or hydroxyl peroxide, which can damage normal tissues [96]. While the cytotoxic effect of TPZ is dependent on low oxygen tensions, tumour reperfusion (represented by tissue diffusion coefficients) and K values (the oxygen concentration is changed to halve the cytotoxic potency) also play a role in the prodrug activation and efficacy [97,98,99].
TPZ was found to have an additive cell killing effect with radiation and cisplatin tumour cell survival [100,101,102]. When given with cisplatin, which mainly targets normoxic tumour cells, TPZ increased therapeutic efficacy. Moreover, TPZ did not potentiate the cisplatin’s toxicity to normal tissues, which made it a potential candidate for clinical trials [103]. Phase I clinical trials of HNSCC, cervical cancer, and limited-stage SCLC showed promising results with the combination treatment of TPZ with cisplatin and radiation, as well as etoposide in the case of SCLC, decreasing the hypoxic tumour areas in patients [104,105,106,107]. Some trials showed the limited efficacy of TPZ in solid tumours. However, the majority of Phase I clinical trials suggested that TPZ, in combination with chemotherapy and radiation, compared to TPZ alone, had the potential to provide better tumour control [108,109]. The dose escalation studies for the TPZ showed cytotoxicity ranging from muscle cramping, vomiting and nausea, febrile neutropenia, and reversible deafness [108,109,110,111,112,113,114].
Phase II trials of TPZ also showed effectiveness when combined with cisplatin, radiation, or gemcitabine in HNSCC, non-small cell lung cancer (NSCLC), advanced or recurrent cervical cancer, and metastatic melanoma [110,111,112,113,114,115,116]. Additionally, the limited stage SCLC trial where TPZ was tested with etoposide, cisplatin and radiation, resulted in a better overall survival [117]. In contrast, a Phase II trial using radiation with TPZ on glioblastoma multiform did not show any differences in survival, indicating that there might be a tumour-type dependent action of TPZ [118].
Phase III clinical trials of cervical carcinoma did not find that TPZ and cisplatin to improve overall survival or progression free survival compared to cisplatin alone in stage IB2, IIA, IIB, IIIB, and IVA patients [119]. The advanced HNSCC trials also resulted in non-statistically significant differences between the combination therapy of cisplatin, TPZ and radiation and the combination of cisplatin and radiation [120]. A Phase III trial of the combination of cisplatin and TPZ in subjects with advanced, previously untreated, non-small cell lung tumours (CATAPULT I) showed a higher survival for the combination of TPZ and cisplatin compared to cisplatin alone. In contrast, the CATAPULT II trial showed that the combination of TPZ and cisplatin was less effective than the combination of etoposide and cisplatin [121,122]. Additionally, the CATAPULT II trial showed that the TPZ group also had higher toxicity. The results of Phase III trials were underwhelming and showed no benefit in having TPZ included in the treatment of the aforementioned cancer types. Furthermore, some even showed a higher toxicity when TPZ was included. However, it needs to be noted that none of these trials fully determined whether TPZ treatment promoted tumour oxygenation, which questions whether the drug was delivered properly and whether it can target hypoxic tumour cells effectively.
6. Hypoxia-Inducible Factor (HIF) Inhibitors
At the molecular level, hypoxia stabilises the hypoxia-inducible transcription factor (HIF), which regulates more than 100 genes involved in tumour angiogenesis, apoptosis, invasion, metastasis and metabolism [135,136]. HIF consists of α and β subunits. Unlike the HIF-β subunit, which is constitutively active, the expression of the HIF-α subunit is regulated by oxygen, iron, free radicals and growth factors. Under normoxic conditions, HIF-α is hydroxylated by prolyl-4-hydroxylase (PHD) and bound to the von Hippel Lindau protein (VHL) to undergo proteasomal degradation by forming the E3 ligase complex [137,138,139]. Hypoxia (but also free radicals or metabolic intermediates) inhibits PHD activity, and thus stabilises HIF-α. When stabilised, HIF-α forms a heterodimer with β and binds to the hypoxia response element (HRE) with cofactors CBP and p300 to transactivate the expression of many downstream genes [140].
HIF pathways are highly upregulated in most tumour types and their expression is correlated with poor patient outcomes [135,141,142,143]. The expression of HIF-α is also upregulated after radiation or chemotherapy, indicating that HIF pathways are significantly involved in treatment resistance [144,145,146,147,148]. Therefore, there is a strong rationale to target HIF pathways to sensitise hypoxic tumour cells to treatment.
This entry is adapted from the peer-reviewed paper 10.3390/biom11111604
This entry is offline, you can click here to edit this entry!
