The formation of severe scars still represents the result of the closure process of extended and deep skin wounds. To address this issue, different bioengineered skin substitutes have been developed but a general consensus regarding their effectiveness has not been achieved yet. It will be shown that bioengineered skin substitutes, although representing a valid alternative to autografting, induce skin cells in repairing the wound rather than guiding a regeneration process. Repaired skin differs from regenerated skin, showing high contracture, loss of sensitivity, impaired pigmentation and absence of cutaneous adnexa (i.e., hair follicles and sweat glands). This leads to significant mobility and aesthetic concerns, making the development of more effective bioengineered skin models a current need. The objective of this review is to determine the limitations of either commercially available or investigational bioengineered skin substitutes and how advanced skin tissue engineering strategies can be improved in order to completely restore skin functions after severe wounds.
- skin substitutes
- tissue engineering
- wound healing
- extracellular matrix
- bottom-up tissue engineering
- vascularization
- bioreactors
- dermal substitutes
- scar tissue.
Pre–Vascularization of Dermis Substitutes
The treatment and the evolution of deep wounds due to thermal burns is schematised in Figure 1A-D. After the debridement of the wound, the bed is filled with a Dermal Regeneration Template (DRT) supporting an artificial layer of silicone-based epidermis. After a period of four weeks, the epidermal layer is detached and an autologous STSG is applied. In a clinical study that used Integra® as the DRT, 20 patients presenting deep wounds were treated using the procedure described in Figure 1A-D. The evolution of the wound was analysed by means of histology, immunocytochemistry and the Vancouver Scar Scale [[1]]. It was observed that the vascularization of the DRT played a crucial role in the take of the STSG. For instance, if the STSG was applied after two or three weeks, the take rate was very low. On the contrary, if the STSG was applied after the fourth week, the take increased up to 95%. Histological and immunostaining analyses demonstrated that at two weeks the vascularization of DRT was poor but increased four weeks after implantation. These data suggest that vascularisation of the DRT and the take of the STSG are strictly related [[1]].
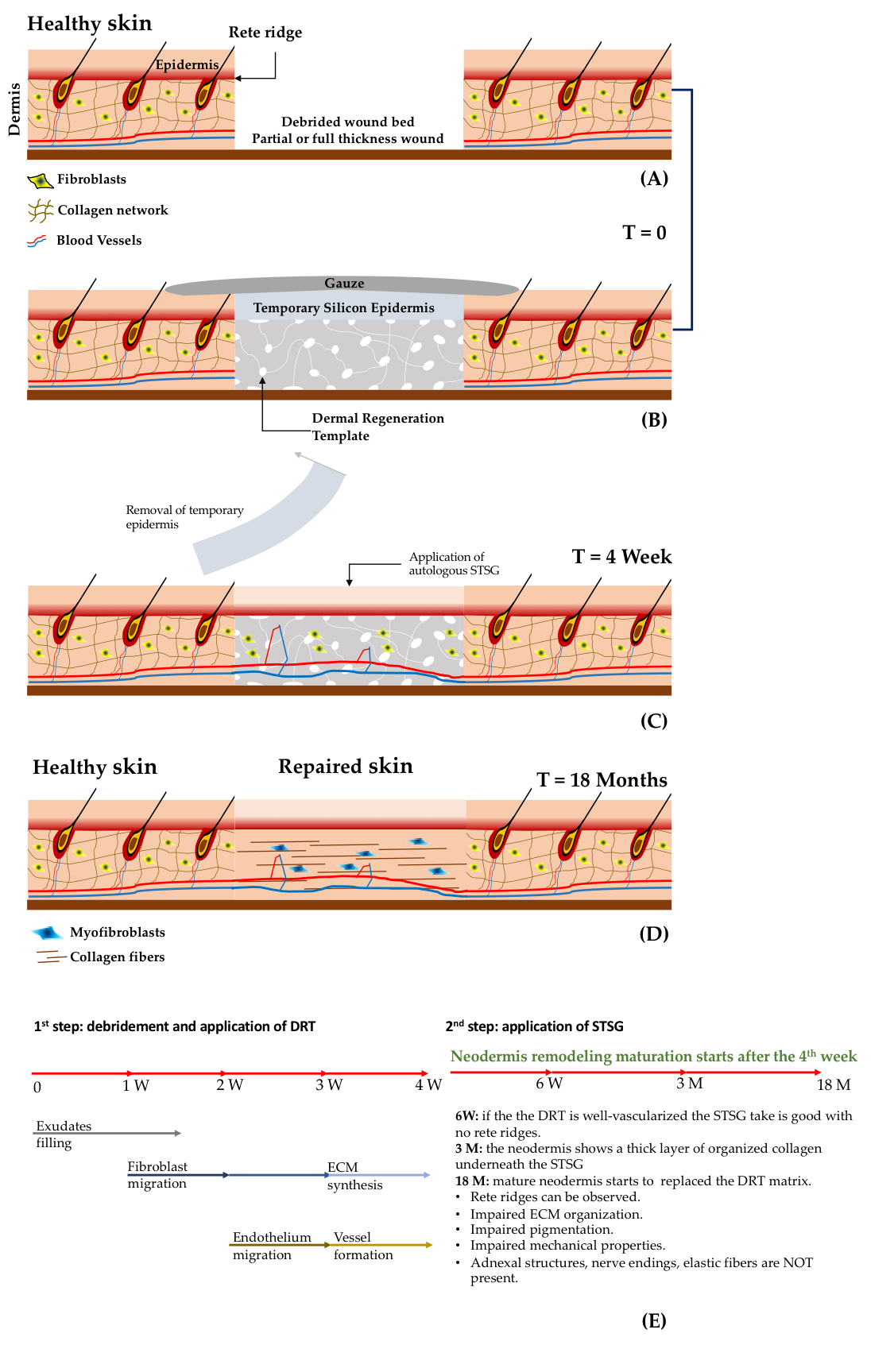
Other relevant findings concern the evolution of the dermis compartment over the time. Weekly histological investigation revealed that influx of exudates and host fibroblasts occurred during weeks one–two. At three weeks, the influx of endothelial cells and the synthesis of immature extracellular matrix components by fibroblasts began. During week four, the formation of a capillary network (Figure 1E) was observed. After the application of the STSG, the wound continued its evolution: at week six a well-organized capillary network was observed, but the dermis–epidermis interface presented no rete ridge profile; at month three, a layer of endogenous collagen network was observed underneath the STSG; after two months, the wound was completely repaired but the neo-tissue was different from the healthy skin. Finally, the complete substitution of the initial DRT with the neodermis occurred at two years post implantation. Even though the patients recovered partial mobility of the damaged parts, it was observed that the repaired zone showed an impaired pigmentation, the mechanical properties between healthy and repaired sites were different, and the organization of the collagen network of the neodermis was different than that of the collagen in the healthy dermis. Finally, neither elastin nor adnexa were present, and differentiation of fibroblasts in myofibroblasts was observed. On the basis of such findings two main issues affecting the DRT emerge: (i) the lack of vascularization [[2],[3]]; and (ii) the limited capability in inducing regeneration instead of repairing processes [[4]]. The take of the STSG has huge implications related to the repairing process, patient mortality, and morbidity and healthcare costs. Indeed, a low take percentage increases the number of re-grafts and the risk of infection by causing either the death of the patient or an increase of hospitalization time in case of morbidity. To increase the take of STSGs, new emerging strategies involve the use of pre-vascularized DRTs [[2],[3],[5],[6],[7]]. By seeding a DRT with adipose tissue-derived microvasculature fragments, a faster vascularization after implants was observed [[8]]. Complete reperfusion of the DRT occurred at day six. The percentage of the take was high if the STSG was applied just after day six, indicating that reperfusion rather than simple vascularization played a crucial role in the take. These data suggest that pre-vascularization of the DRT can contribute to shortening the timeframe needed for the application of an STSG. On the other hand, a one-step surgery, which may decrease the number of surgical operations, cannot be performed yet. To do this, not only vascularization, but also fast reperfusion should be promoted.
Engineered Skin Composed of Fibroblast-Assembled Extracellular Matrix
The lack of vascularization at the moment of implantation has been recognized as the main issue affecting the take of the STSG. No studies have been performed yet on the role that the extracellular matrix comprising the DRT may play on both vascularization and longtime dermal remodeling [[9]]. The dermis compartment of the totality of the skin substitutes (either cellularized or acellular) are composed by exogenous extracellular materials, i.e., not assembled by the fibroblasts of the patient. This should represent the limitation of the currently available tissue engineering skins. Indeed, exogenous matrices, even though of natural origins, cannot fully replicate the complexity of the living dermis. This may ultimately compromise the repository and regulatory role that the native cell-assembled extracellular matrix plays [[9],[10], [11]]. Such a mismatch between an exogenous material and the living dermis may be responsible for the impaired repair process at both cellular and extracellular levels. Firstly, because the repository and regulatory role of the native ECM is depressed, the growth factors secreted by fibroblasts are not correctly presented to other cell types (e.g., keratinocytes and endothelial cells) neither in space nor time, generating a possible “mistake” in cell–cell signalling. This could explain both the delay in the vascularization time and the delayed formation of the rete ridge profile at dermal epidermal interfaces [[1],[12],[13]]. As confirmation of this, in vitro tissue engineered skin made by exogenous natural hydrogels (i.e., collagen, fibrin, etc.) presents a flat dermal–epidermal interface. On the contrary, if epithelial cells are grown on a fibroblast-assembled ECM, it is possible to observe a rete ridge profile with spontaneous formation of epithelial invagination and follicular-like structures (Figure 1C) [[14]], which are typical of the physiologic dermal–epidermal cross-talk mediated by the extracellular matrix [[14]]. The lack of endogenous ECM-mediated signaling may also explain the absence of both cutaneous adnexa and nerve endings in repaired deep wounds [[1],[12],[15]]. Secondly, when fibroblasts colonize the inner porosity of the DRT, they produce an immature extracellular matrix with a degree of assembly much lower than the degree of assembly of the surrounding healthy dermis. Such an immature protein network is not able to withstand the traction forces of the fibroblasts [[16],[17]], generating a different architecture of the collagen fibers in the wound compared to the healthy dermis [[18],[19],[20],77]. Macroscopically, these phenomena generate a portion of the cutis possessing different mechanical properties, different pigmentation, absence of sensing properties and high contracture, provoking both severe functional and aesthetic concerns.
To overcome such limitations, a tissue engineering strategy to produce a human dermis substitute composed of a fibroblast-assembled extracellular matrix has been developed [[13],[21]]. The innovative idea of such strategy is to let human fibroblasts producing their own ECM in vitro. This process provides the possibility of modulating the properties of the cell-synthesized ECM, in order to obtain a final dermis having both composition and assembly degree of the collagen network relatively similar to those present in vivo. Moreover, no exogenous materials are present. This bottom-up tissue engineering strategy starts with the fabrication of dermal building blocks obtained [[22]] by seeding human fibroblasts in porous gelatin microspheres (figure 2A). It has been demonstrated that by optimizing the culture conditions, the fibroblasts can produce their own extracellular matrix. Such building blocks, named Dermal-µTissues, were subsequently moulded and packed in maturation chambers where both cell–cell and ECM–ECM interactions took place, leading to the formation of a continuum, up to 2 mm thick, made of an endogenous dermis containing fibroblasts and gelatin microspheres. By modulating the stiffness and the degradation rate of the gelatine microspheres and by engineering the dynamic culture conditions (Figure 2), it was possible to obtain fine control over the maturation status and assembly of both collagen and elastin networks [[16],[23]]. During the duration of the process (approximately five weeks), gelatin microspheres were degraded by protease digestion and the final tissue, named EndoDermis, was completely made up of fibroblasts embedded in their own extracellular matrix (Figure 2A). Interestingly, the collagen network was characterized by a stiffness and degree of assembly similar to that featuring the human skin. In the ECM elastin, hyaluronic acid, fibronectin and elastin were also present (Figure 2B-F). In order to produce a pre-vascularized endogenous human dermis model, human umbilical vein endothelial cells (HUVECs) were seeded on the EndoDermis and it was allowed to form an interconnected capillary network [[6]] that occurred within three weeks (Figure 2D, E). At the best of our knowledge, other than a capillary network, such an engineered DRT is the first model completely formed by a fibroblast-assembled extracellular matrix [[6]]. After subcutaneous implant in a nude mouse model, fibroblasts and their own ECM (the neodermis) were already present and well-assembled. Thus, no additional time is required for fibroblast influx and neodermis formation. The only phenomenon required is the anastomosis and perfusion of the engineered capillary network. This was shown to occur within seven days of implantation (Figure 2H). Although further investigations are currently being conducted of a more representative wound model, such data are encouraging. In addition to vascularization, which has been recognized as a critical issue affecting the effectiveness of a DRT, the described tissue engineered strategy allows the fabrication of a DRT composed of a native extracellular matrix starting from a small number of fibroblasts derived from the patient. In this way, the risks associated with the allogenic nature of the cells and the impaired ECM assembly during wound closure, can be drastically reduced. According to this idea, the formation of severe scars can be reduced.
![The main steps for the production of a DRT composed of fibroblast-assembled/prevascularized human dermis substitutes, and its morphological features before and after implantation in a nude mice model. (A) From left to right: production of Dermal-Tissues; their molding and assembly in a maturation chamber that is kept under dynamic culture conditions; formation of a continuum of fibroblasts embedded in their own dermal extracellular matrix; epithelization and vascularization of the endogenous human dermis. (B) Fabrication of large pieces of endogenous human dermis (major dimension 20 cm). (C) Histology of the endogenous human dermis supporting the differentiation of epidermis with the formation of spontaneous rete ridge profile. (D) Vascularized endogenous human dermis: cell nuclei in green and capillary network in red. (E) Vascularized endogenous human dermis: fibroblast-assembled collagen bundles observed under label-free multiphoton microscopy in gray; capillary network in red. (F) Top: fibroblast-assembled hyaluronic acid in green, cell nuclei in blue; Bottom: fibroblast-assembled elastin network in yellow, cell nuclei in blue. (G) Implantation of a piece of the pre-vascularized endogenous human dermis. (H) Connection between engineered capillary network (green) and recipient capillary network (red); fibroblast-assembled collagen in gray. Figure 3B, 3D, 3E, 3G, and 3H are from reference [34] “Mazio, C. et al. Pre-vascularized dermis model for fast and functional anastomosis with host vasculature. Biomat. 192, 159–170 (2019)”. Authors obtained permision from Elsevier: License Number 4681910194044.](https://encyclopedia.pub/revision/image/dba15f31d4f1b05832b4f533881ca4b8/5de4e920cf355.png)
References
- Naiem S. Moiemen; Evangelia Vlachou; Jonathan J. Staiano; Yi Thawy; James D. Frame; Reconstructive Surgery with Integra Dermal Regeneration Template: Histologic Study, Clinical Evaluation, and Current Practice. Plastic and Reconstructive Surgery 2006, 117, 160S-174S, 10.1097/01.prs.0000222609.40461.68.
- Florian S. Frueh; Nadia Sanchez-Macedo; Maurizio Calcagni; Pietro Giovanoli; Nicole Lindenblatt; The Crucial Role of Vascularization and Lymphangiogenesis in Skin Reconstruction. European Surgical Research 2018, 59, 242-254, 10.1159/000492413.
- M.W. Laschke; M.D. Menger; Vascularization in Tissue Engineering: Angiogenesis versus Inosculation. European Surgical Research 2012, 48, 85-92, 10.1159/000336876.
- Makoto Suzuki; Nayuta Yakushiji; Yasuaki Nakada; Akira Satoh; Hiroyuki Ide; Koji Tamura; Limb Regeneration in Xenopus laevis Froglet. TSW Development & Embryology 2006, 1, 26-37, 10.1100/tswde.2006.114.
- Anna Domaszewska-Szostek; Marta Krzyżanowska; Maria Siemionow; Cell-Based Therapies for Chronic Wounds Tested in Clinical Studies. Annals of Plastic Surgery 2019, 83, e96-e109, 10.1097/sap.0000000000001947.
- Claudia Mazio; Costantino Casale; Giorgia Imparato; Francesco Urciuolo; Chiara Attanasio; Maria De Gregorio; Francesca Rescigno; Paolo A. Netti; Pre-vascularized dermis model for fast and functional anastomosis with host vasculature. Biomaterials 2019, 192, 159-170, 10.1016/j.biomaterials.2018.11.018.
- Francesca Martorina; Costantino Casale; Francesco Urciuolo; Paolo A. Netti; Giorgia Imparato; In vitro activation of the neuro-transduction mechanism in sensitive organotypic human skin model. Biomaterials 2017, 113, 217-229, 10.1016/j.biomaterials.2016.10.051.
- Florian S. Frueh; Thomas Später; Christina Körbel; Claudia Scheuer; Anna C. Simson; Nicole Lindenblatt; Pietro Giovanoli; Michael D. Menger; Matthias W. Laschke; Prevascularization of dermal substitutes with adipose tissue-derived microvascular fragments enhances early skin grafting.. Scientific Reports 2018, 8, 10977, 10.1038/s41598-018-29252-6.
- Tania Rozario; Uglas W. DeSimone; The extracellular matrix in development and morphogenesis: a dynamic view.. Developmental Biology 2009, 341, 126-40, 10.1016/j.ydbio.2009.10.026.
- Stephanie L.K. Bowers; Indroneal Banerjee; Troy A. Baudino; The extracellular matrix: at the center of it all.. Journal of Molecular and Cellular Cardiology 2009, 48, 474-82, 10.1016/j.yjmcc.2009.08.024.
- Fiona M. Watt; Hironobu Fujiwara; Cell-Extracellular Matrix Interactions in Normal and Diseased Skin. Cold Spring Harbor Perspectives in Biology 2011, 3, a005124-a005124, 10.1101/cshperspect.a005124.
- Heli Lagus; Maarit Sarlomo-Rikala; Tom Böhling; Jyrki Vuola; Prospective study on burns treated with Integra®, a cellulose sponge and split thickness skin graft. Burns 2013, 39, 1577-1587, 10.1016/j.burns.2013.04.023.
- David Vader; Alexandre Kabla; David Weitz; Lakshminarayana Mahadevan; Strain-Induced Alignment in Collagen Gels. PLOS ONE 2009, 4, e5902, 10.1371/journal.pone.0005902.
- Costantino Casale; Giorgia Imparato; Francesco Urciuolo; Paolo A. Netti; Endogenous human skin equivalent promotes in vitro morphogenesis of follicle-like structures. Biomaterials 2016, 101, 86-95, 10.1016/j.biomaterials.2016.05.047.
- Ivan B. Wall; Ryan Moseley; Duncan M. Baird; David Kipling; Peter Giles; Iraj Laffafian; Patricia E. Price; David W. Thomas; Phil Stephens; Fibroblast Dysfunction Is a Key Factor in the Non-Healing of Chronic Venous Leg Ulcers. Journal of Investigative Dermatology 2008, 128, 2526-2540, 10.1038/jid.2008.114.
- Giorgia Imparato; Francesco Urciuolo; Costantino Casale; Paolo A. Netti; The role of microscaffold properties in controlling the collagen assembly in 3D dermis equivalent using modular tissue engineering. Biomaterials 2013, 34, 7851-7861, 10.1016/j.biomaterials.2013.06.062.
- Cécile Philandrianos; Lucile Andrac-Meyer; Serge Mordon; Jean-Marc Feuerstein; Florence Sabatier; Julie Veran; Guy Magalon; Dominique Casanova; Comparison of five dermal substitutes in full-thickness skin wound healing in a porcine model. Burns 2012, 38, 820-829, 10.1016/j.burns.2012.02.008.
- T Velnar; T Bailey; V Smrkolj; The Wound Healing Process: An Overview of the Cellular and Molecular Mechanisms. Journal of International Medical Research 2009, 37, 1528-1542, 10.1177/147323000903700531.
- Bernadette Lombardi; Costantino Casale; Giorgia Imparato; Francesco Urciuolo; Paolo Antonio Netti; Spatiotemporal Evolution of the Wound Repairing Process in a 3D Human Dermis Equivalent. Advanced Healthcare Materials 2017, 6, 1601422, 10.1002/adhm.201601422.
- Clark JA; Mechanical properties of normal skin and hypetrophic scars. Burns 1996, 11, 443, 10.1016/0305-4179(96)00038-1.
- Mathew Varkey; Jie Ding; Edward E. Tredget; Advances in Skin Substitutes—Potential of Tissue Engineered Skin for Facilitating Anti-Fibrotic Healing. Journal of Functional Biomaterials 2015, 6, 547-563, 10.3390/jfb6030547.
- F Urciuolo; A Garziano; Giorgia Imparato; V Panzetta; S Fusco; C Casale; Paolo Netti; Biophysical properties of dermal building-blocks affect extra cellular matrix assembly in 3D endogenous macrotissue. Biofabrication 2016, 8, 015010, 10.1088/1758-5090/8/1/015010.
- Karoly Jakab; Cyrille Norotte; Brook Damon; Francoise Marga; Adrian Neagu; Cynthia L. Besch-Williford; Anatoly Kachurin; Kenneth H. Church; Hyoungshin Park; Vladimir Mironov; et al. Tissue Engineering by Self-Assembly of Cells Printed into Topologically Defined Structures. Tissue Engineering Part A 2008, 14, 413-421, 10.1089/tea.2007.0173.
