Lipid oxidation (LO) is a primary cause of quality deterioration in fat-containing dairy powders and is often used as an estimation of a products shelf-life and consumer acceptability. The LO process produces numerous volatile organic compounds (VOC) including aldehydes, ketones and alcohols, which are known to contribute to the development of off-flavours in dairy powders. The main factors influencing the oxidative state of dairy powders and the various analytical techniques used to detect VOC as indicators of LO in dairy powders are outlined.
- lipid oxidation
- dairy powder
- sensory
1. Introduction
Oxidation of bovine milk fat is recognised as the main factor in the development of undesirable flavours in products such as whole milk powder (WMP) and infant milk formula (IMF). Lipid oxidation (LO) is responsible for the formation of primary and secondary oxidation products including aldehydes, ketones and alcohols, which can impact on nutritional and sensory properties of dairy powders [1]. Factors that can contribute to the oxidative stability of dairy powders include the quality of the raw milk (fatty acid (FA) composition, bovine diet, and storage conditions), processing parameters, powder composition (especially water activity), presence of pro- and anti-oxidants (natural or added during processing), packaging materials, storage and transport conditions. LO is a free radical chain reaction consisting of three stages; initiation, propagation, and termination. Free radicals and peroxides (tasteless, flavourless compounds) [2] are generated during the initiation phase when molecular oxygen reacts with unsaturated FA [3]. The rate of the propagation cycle is directly proportional to the degree of lipid unsaturation [4]. The resultant termination products (secondary LO products) are generally quite stable, however, it is these secondary LO products (mainly aldehydes, ketones, alcohols, and hydrocarbons) that actually contribute to off-flavour development and have been described as grassy, soapy, cardboard-like, painty, tallowy and/or fishy [5][6]. Secondary LO compounds can be monitored and quantified instrumentally as molecular-level indicators of oxidised flavours in dairy products. This measurement can be used in place or in combination with sensory analysis to provide an overall profile of the flavour stability of a dairy powder [6][7]. The presence and increase of numerous secondary LO products in dairy powders during processing and storage is well documented [6][8][9]. However, there is a lack of knowledge linking the quantification of volatiles associated with LO to descriptive sensory attributes in dairy products in general [10]. The aims of this review are as follows: (1) to summarize the main factors influencing the oxidation of dairy powders, (2) to summarise the various analytical techniques used to detect and quantify VOC as indicators of LO, and (3) to highlight the use of combined analytical and sensory approaches to better understand the LO process in dairy powders.
2. Mechanism of Lipid Oxidation
It is generally accepted that oxygen reacts naturally with many organic substrates resulting in the formation of primary oxidation products; hydroperoxides and other oxygenated compounds. There are three known types of LO that can affect dairy products; auto-oxidation, photo-oxidation, and metal induced oxidation [11].
The mechanism of the auto-oxidation of PUFA as a radical chain reaction was established more than half a century ago. The process of LO can be broken into three distinct, but partially overlapping phases of radical reactions; initiation, propagation and termination [12] ( Figure 1 , Figure 2 and Figure 3 ). Free radicals and peroxides, both of which are highly reactive, are generated during the initiation phase when molecular oxygen reacts with unsaturated FA. In addition to oxygen, oxidative initiators such as chemical oxidisers, transition metals (e.g., copper and iron), and enzymes (e.g., lipoxygenases) contribute to the rate of the initiation phase [3]. Heat and light also exacerbate the rate of the initiation phase and the other phases of LO [13]. The rate of auto-oxidation is increased by increasing unsaturation of the alkyl chain [13], and the matrix also plays a role in the susceptibility of a product to oxidation [14]. FA alkyl chains are susceptible to oxidation at alkene bonds and neighbouring allylic carbons.
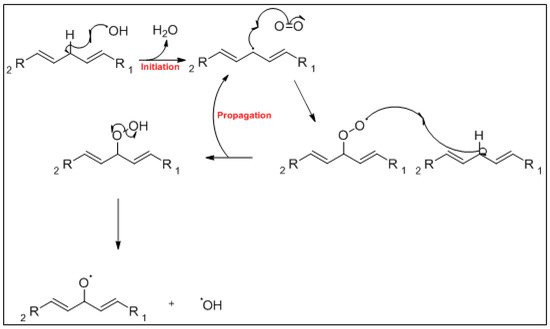
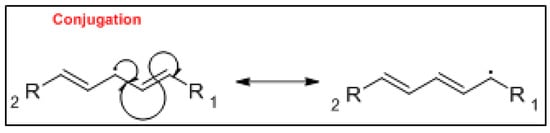
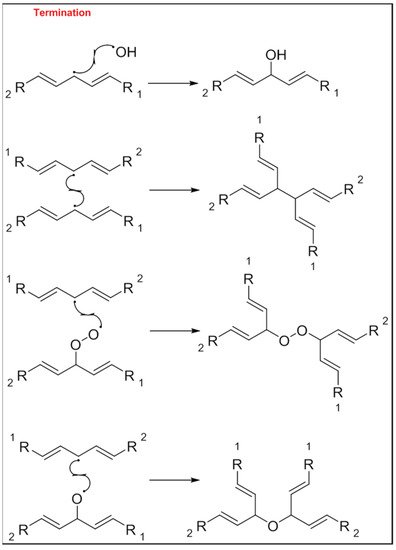
Photo-oxidation and free-radical reactions at allylic carbons are responsible for the breakdown of unsaturated lipids [12][13][15]. These reactions produce hydroperoxides in these allylic bonds, and cause changes in the position and geometry of double bonds. Auto-oxidation and photo-oxidation are associated with different hydroperoxide reaction products, indicating that different reaction mechanisms are involved [16]. Photo-oxidation of milk has been well documented [11][17], exposure to light, either natural or artificial, can cause development of off-flavours in milk within 15 min [18]. The subsequent aromas have been characterised as burnt protein, cabbage-like and plastic [11], however, their intensity can decrease the longer the milk is exposed to light, allowing newly activated off-flavours to dominate. These off-flavours have been described as cardboard-like, metallic and rancid [19][20][21]. Exposure to ultra-violet (UV) light can enable the oxidation of fat to volatile aldehyde compounds and has also been found to cause the degradation of sulfur–containing compounds, both of which are major contributors to off-flavours in milk [18]. A study by Silcock et al. [22] reported good correlation between negative sensory perceptions and VOC formation for milk stored in light-exposed containers, these include photo-oxidation and auto-oxidation compounds such as dimethyl disulfide, and aldehydes such as heptanal, pentanal and hexanal. For milk stored in containers protected from light, no correlation between the sensory attributes and VOC was documented.
Furthermore, the type of light the product is exposed too can also have an impact on the levels of oxidation. A study by Brothersen et al. [18] demonstrated that exposure of milk to fluorescent light (commonly used in the retail of dairy products) resulted in greater changes in LO levels, compared with exposure to white light-emitting diodes (LED). This study demonstrated that even high quality milk is susceptible to photo-oxidation at the point of sale dependent upon the type of lighting.
3. Secondary Reactions Associated with Lipid Oxidation
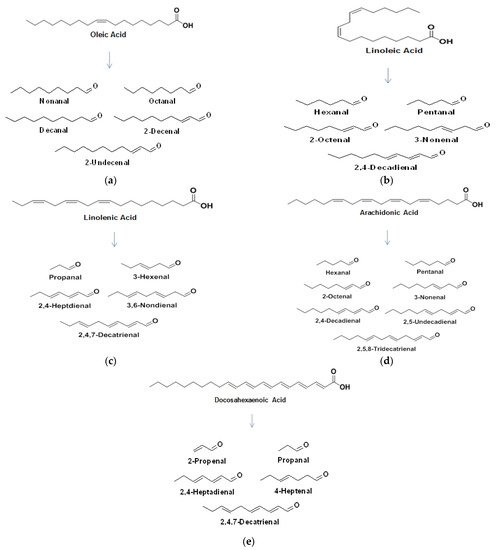
Various FA within milk are broken down via oxidation to primary and secondary oxidation products. The formation of a hydroxyperoxide through the oxidative mechanisms discussed earlier, breaks down to form an alkoxyl radical which splits by homolytic β-scission each side of the carbon bonded to the oxygen radical. The major FA in milk and some of their associated breakdown products are outlined in Figure 4 a–e.
Along with LO, the Maillard reaction is an important chemical reaction that occurs in numerous foods, and both reactions have been shown to influence each other [23]. The Maillard reaction is a well-documented, non-enzymatic browning reaction between the amine groups of free amino acids, peptides or proteins and reactive carbonyl groups of reducing sugars under thermal processing and/or storage conditions [24]. This reaction can occur at room temperature, but is optimal at much higher temperatures (140–165 °C). The Maillard reaction has been identified as a main factor in quality deterioration of IMF [25]. However, in whey protein concentrate (WPC) and whey protein isolate (WPI), Maillard reaction products contribute to a lesser extent to flavour formation than LO [26][27]. The moisture content must be below 3% w / w for the Maillard reaction to conclude, a value that is not reached in most dried dairy products [28]. The Maillard reaction mechanism is outlined in Figure 5 .
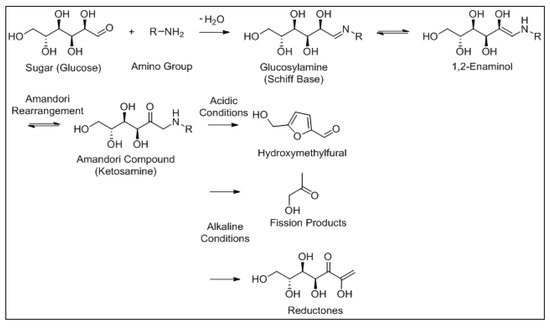
Similar to the Maillard reaction, the Strecker reaction mechanism is also linked to LO. Aldehydes are readily converted to secondary alcohols or acids and are therefore known as transitory volatile compounds with some known to be a result of Strecker reactions [29][30]. The degradation of amino acids during the Strecker reaction is one of the primary mechanisms resulting in the final aroma compounds of the Maillard reaction. The process involves the oxidative deamination and decarboxylation of the amino acid in the presence of α-dicarbonyl compounds formed in the Maillard reaction and the formation of the corresponding Strecker aldehyde [31][32]. Each amino acid produces a specific Strecker aldehyde which comprises one carbon atom less than the amino acid from which it is formed. Strecker aldehydes such as 3-methylbutanal (malty flavour) [33] and phenylacetaldehyde (honey-like flavour) are derived from leucine and phenylalanine, respectively, and are commonly reported as aroma contributors in dairy products [34]. LO and Maillard reactions interact in complex food systems and can share common chemical mechanisms and intermediate compounds [23]. Moreover, certain carbonyls derived from LO such as alkadienals and ketodienes have been shown to promote the oxidative degradation of amino acids to produce the corresponding Strecker aldehydes via Strecker-type reactions [35][36]. The Strecker reaction is outlined in Figure 7 .
4. Qualitative and Quantitative Measurement of Lipid Oxidation Compounds in Dairy Products
There are various techniques and strategies used to measure LO in dairy products. Some commonly used, relatively simple, and practical methods to assess LO are POV, TBARS, and the KREIS test. Their widespread use is mainly due to ease of use and low cost, although they are more qualitative rather than quantitative.
Several analytical methods have been optimised for detecting off-flavours associated with LO in dairy products, such as solvent-assisted flavour evaporation (SAFE), GC-MS [37], and GC-O [38]. GC-flame ionization detection (FID) or GC-MS have become the methods of choice for quantitative VOC analysis. These approaches are undertaken in combination with a specific method to extract and concentrate the VOC using either static or dynamic headspace techniques, sorption-based techniques, liquid based extraction or solvent assisted techniques. As previously mentioned, care must be taken not to increase VOC associated with LO during the analytical technique, as previous studies have demonstrated that certain LO VOC can increase between 37 °C and 60 °C [39], therefore, including appropriate controls is necessary to prevent false positives.
TD also works on the bases of heating samples to allow VOC reach the gaseous phase. As with HS-SPME, TD is used as an extraction and pre-concentration step prior to analysis by GC. VOC and some semi-VOC are extracted by this technique onto suitable phases packed into TD tubes, with many different phases available that can target individual VOC or chemical classes, or for more generic untargeted approaches. Removal of the trapped compounds from the phase(s) onto the GC column involves heating of the TD tube in a gas flow, and sometimes further concentration is possible using an in-line focusing trap. TD has been applied to a number of dairy products [40][41], milk powder [42], and IMF [43]. A number of application notes are also available on the use of TD [44][45][46]. Its widespread use in dairy products may be limited as moisture management can be problematic.
Faulkner et al. [40] achieved good results for milk samples (up to 65 volatile compounds from a range of chemical classes) using a new high capacity SE technique called HiSorb followed by GC-MS analysis. Currently PDMS-coated stir-bars are the only phase commercially available for these techniques, which somewhat reduces the applicability of SBSE to the extraction of non-polar compounds due to the poor extractability of more polar analytes [47]. However, Ochiai et al. [48] demonstrated that solvent-assisted SBSE improves peak resolution and extraction efficiency of polar and non-polar compounds. Moreover, Schiano et al. [49] concluded that solvent-assisted SBSE provided the most consistent detection of selected compounds in commercial milks, although the levels of compounds detected were not significantly ( p > 0.05) higher compared to conventional SBSE or SPME extraction methods. Some of the most common techniques used for the extraction of volatiles from dairy powders are outlined in Table 1 .
| Method | Advantages | Limitations | Applications | Reference |
|---|---|---|---|---|
| Extraction Methodology | ||||
| Headspace solid-phase microextraction (HS-SPME) |
|
|
|
[50][51][52][53][54][55] |
| In-tube extraction (ITEX) |
|
|
|
[56] |
| Thermal desorption (TD) |
|
|
|
[40][41][42][44][45][46] |
| Solvent-assisted flavour evaporation (SAFE) |
|
|
|
[57] |
| Stir bar sorptive extraction (SBSE) |
|
|
|
[45][58][48][59] |
| HiSorb extraction |
|
|
|
[60] |
| Identification Methodology | ||||
| Mass Spectrometry (MS) |
|
|
|
[61][62][63] |
| Flame Ionised Detector (FID) |
|
|
|
[64] |
| Gas chromatography olfactometry (GC-O) |
|
|
|
[65] |
| GCxCG-ToF-MS (Time of Flight-MS) |
|
|
|
[66] |
This entry is adapted from the peer-reviewed paper 10.3390/foods10102315
References
- Boroski, M.; Giroux, H.J.; Sabik, H.; Petit, H.V.; Visentainer, J.V.; Matumoto-Pintro, P.T.; Britten, M. Use of oregano extract and oregano essential oil as antioxidants in functional dairy beverage formulations. LWT—Food Sci. Technol. 2012, 47, 167–174.
- Kochhar, S. Oxidative pathways to the formation of off-flavours. In Food Taints and Off-Flavours; Springer: Berlin/Heidelberg, Germany, 1996; pp. 168–225.
- Kolanowski, W.; Jaworska, D.; Weißbrodt, J. Importance of instrumental and sensory analysis in the assessment of oxidative deterioration of omega-3 long-chain polyunsaturated fatty acid-rich foods. J. Sci. Food Agric. 2007, 87, 181–191.
- Kubow, S. Routes of formation and toxic consequences of lipid oxidation products in foods. Radic. Biol. Med. 1992, 12, 63–81.
- Li, Y.; Wang, W. Formation of oxidized flavor compounds in concentrated milk and distillate during milk concentration. J. Dairy Sci. 2016, 99, 9647–9651.
- Lloyd, M.; Drake, M.; Gerard, P. Flavor Variability and Flavor Stability of US-Produced Whole Milk Powder. J. Food Sci. 2009, 74, S334–S343.
- Romeu-Nadal, M.; Chavez-Servin, J.; Castellote, A.; Rivero, M.; Lopez-Sabater, M. Oxidation stability of the lipid fraction in milk powder formulas. Food Chem. 2007, 100, 756–763.
- Hall, G.; Andersson, J.; Lingnert, H.; Olofssono, B. Flavor changes in whole milk powder during storage. J. Food Qual. 1985, 7, 153–190.
- Hougaard, A.B.; Vestergaard, J.S.; Varming, C.; Bredie, W.L.; Ipsen, R.H. Composition of volatile compounds in bovine milk heat treated by instant infusion pasteurisation and their correlation to sensory analysis. Int. J. Dairy Technol. 2011, 64, 34–44.
- Kilcawley, K.N.; Faulkner, H.; Clarke, H.J.; O’Sullivan, M.G.; Kerry, J.P. Factors Influencing the Flavour of Bovine Milk and Cheese from Grass Based versus Non-Grass Based Milk Production Systems. Foods 2018, 7, 37.
- Gutierrez, A.M. Effects of Lipid Oxidation Initiators and Antioxidants on the Total Antioxidant Capacity of Milk and Oxidation Products during Storage. Master’s Thesis, Iowa State University, Ames, IA, USA, 2014.
- Shahidi, F.; Zhong, Y. Lipid oxidation: Measurement methods. In Bailey’s Industrial Oil and Fat Products; Wiley: Hoboken, NJ, USA, 2005.
- Frankel, E. Frying fats. In Lipid Oxidation; The Oily Press: Dundee, UK, 1998; pp. 227–248.
- Miyashita, K. Polyunsaturated lipids in aqueous systems do not follow our preconceptions of oxidative stability. Lipid Technol. Newsl. 2002, 8, 35–41.
- Rawls, H.R.; Van Santen, P. A possible role for singlet oxygen in the initiation of fatty acid autoxidation. J. Am. Oil Chem. Soc. 1970, 47, 121–125.
- Simkovsky, N.M.; Ecker, A. Einfluß von Licht und Tocopherolgehalt auf die Oxidationsstabilität von Fettsäuremethylestern. Lipid Fett. 1998, 100, 534–538.
- Wishner, L.A. Light-induced oxidations in milk. J. Dairy Sci. 1964, 47, 216–221.
- Brothersen, C.; McMahon, D.; Legako, J.; Martini, S. Comparison of milk oxidation by exposure to LED and fluorescent light. J. Dairy Sci. 2016, 99, 2537–2544.
- Stull, J. The Effect of Light on Activated Flavor Development and on the Constituents of Milk and its Products: A Review. J. Dairy Sci. 1953, 36, 1153–1164.
- Jung, M.; Yoon, S.; Lee, H.; Min, D. Singlet Oxygen and Ascorbic Acid Effects on Dimethyl Disulfide and Off-Flavor in Skim Milk Exposed to Light. J. Food Sci. 1998, 63, 408–412.
- Hedegaard, R.; Kristensen, D.; Nielsen, J.H.; Frøst, M.B.; Østdal, H.; Hermansen, J.E.; Kröger-Ohlsen, M.; Skibsted, L.H. Comparison of descriptive sensory analysis and chemical analysis for oxidative changes in milk. J. Dairy Sci. 2006, 89, 495–504.
- Silcock, P.; Alothman, M.; Zardin, E.; Heenan, S.; Siefarth, C.; Bremer, P.; Beauchamp, J. Microbially induced changes in the volatile constituents of fresh chilled pasteurised milk during storage. Food Packag. Shelf Life 2014, 2, 81–90.
- Zamora, R.; Hidalgo, F.J. Coordinate contribution of lipid oxidation and Maillard reaction to the nonenzymatic food browning. Crit. Rev. Food Sci. Nutr. 2005, 45, 49–59.
- Chen, X.-M.; Kitts, D.D. Characterization of antioxidant and anti-inflammatory activities of bioactive fractions recovered from a glucose− lysine Maillard reaction model system. Mol. Cell. Biochem. 2012, 364, 147–157.
- Nunes, L.; Martins, E.; Perrone, Í.T.; de Carvalho, A.F. The Maillard Reaction in Powdered Infant Formula. J. Food Nutr. Res. 2019, 7, 33–40.
- Whetstine, M.C.; Croissant, A.; Drake, M. Characterization of dried whey protein concentrate and isolate flavor. J. Dairy Sci. 2005, 88, 3826–3839.
- Tunick, M.H.; Thomas-Gahring, A.; Van Hekken, D.L.; Iandola, S.K.; Singh, M.; Qi, P.X.; Ukuku, D.O.; Mukhopadhyay, S.; Onwulata, C.I.; Tomasula, P.M. Physical and chemical changes in whey protein concentrate stored at elevated temperature and humidity. J. Dairy Sci. 2016, 99, 2372–2383.
- Sienkiewicz, T.; Riedel, C. Whey and Whey Utilization: Possibilities for Utilization in Agriculture and Foodstuffs Production; Verlag Th. Mann: Gelsenkirchen-Buer, Germany, 1990.
- Atasoy, A.F.; Hayaloglu, A.A.; Kırmacı, H.; Levent, O.; Türkoğlu, H. Effects of partial substitution of caprine for ovine milk on the volatile compounds of fresh and mature Urfa cheeses. Small Rumin. Res. 2013, 115, 113–123.
- Kondyli, E.; Massouras, T.; Katsiari, M.; Voutsinas, L. Lipolysis and volatile compounds of Galotyri-type cheese made using different procedures. Small Rumin. Res. 2013, 113, 432–436.
- Mottram, D.S. Flavour formation in meat and meat products: A review. Food Chem. 1998, 62, 415–424.
- Estévez, M.; Ventanas, S.; Heinonen, M. Formation of Strecker aldehydes between protein carbonyls–α-aminoadipic and γ-glutamic semialdehydes–and leucine and isoleucine. Food Chem. 2011, 128, 1051–1057.
- Zhou, Q.; Wintersteen, C.L.; Cadwallader, K.R. Identification and quantification of aroma-active components that contribute to the distinct malty flavor of buckwheat honey. J. Agric. Food Chem. 2002, 50, 2016–2021.
- Delgado, F.J.; González-Crespo, J.; Cava, R.; García-Parra, J.; Ramírez, R. Characterisation by SPME–GC–MS of the volatile profile of a Spanish soft cheese PDO Torta del Casar during ripening. Food Chem. 2010, 118, 182–189.
- Zamora, R.; Gallardo, E.; Hidalgo, F.J. Strecker degradation of phenylalanine initiated by 2,4-decadienal or methyl 13-oxooctadeca-9,11-dienoate in model systems. J. Agric. Food Chem. 2007, 55, 1308–1314.
- Zamora, R.; Gallardo, E.; Hidalgo, F.J. Model studies on the degradation of phenylalanine initiated by lipid hydroperoxides and their secondary and tertiary oxidation products. J. Agric. Food Chem. 2008, 56, 7970–7975.
- Havemose, M.; Justesen, P.; Bredie, W.; Nielsen, J.H. Measurement of volatile oxidation products from milk using solvent-assisted flavour evaporation and solid phase microextraction. Int. Dairy J. 2007, 17, 746–752.
- Zellner, B.d.A.; Dugo, P.; Dugo, G.; Mondello, L. Gas chromatography–olfactometry in food flavour analysis. J. Chromatogr. A 2008, 1186, 123–143.
- Panseri, S.; Soncin, S.; Chiesa, L.M.; Biondi, P.A. A headspace solid-phase microextraction gas-chromatographic mass-spectrometric method (HS-SPME–GC/MS) to quantify hexanal in butter during storage as marker of lipid oxidation. Food Chem. 2011, 127, 886–889.
- Faulkner, H.; O’Callaghan, T.F.; McAuliffe, S.; Hennessy, D.; Stanton, C.; O’Sullivan, M.G.; Kerry, J.P.; Kilcawley, K.N. Effect of different forage types on the volatile and sensory properties of bovine milk. J. Dairy Sci. 2018, 101, 1034–1047.
- Valero, E.; Miranda, E.; Sanz, J.; Martinez-Castro, I. Automatic thermal desorption in GC analysis of dairy product volatiles. Chromatographia 1997, 44, 59–64.
- Francesca, I.; Patrizia, P.; Luca, C.; Federico, M.; Annalisa, R. Analysis of volatile compounds in powdered milk for infant nutrition by direct desorption (CIS4–TDU) and GC–MS. Talanta 2015, 141, 195–199.
- Cheng, H.; Zhu, R.-G.; Erichsen, H.; Soerensen, J.; Petersen, M.A.; Skibsted, L.H. High temperature storage of infant formula milk powder for prediction of storage stability at ambient conditions. Int. Dairy J. 2017, 73, 166–174.
- Roberts, G.; Kelly, L.; Barden, D. Flavour Profiling of Milk and Premium Teas by HiSorb Sorptive Extraction with Thermal Desorption-GC-MS Analysis. LC GC Eur. 2016, 29, 699–700.
- Hoffmann, A.; Heiden, A. Determination of flavor and off flavor compounds in dairy products using stir bar sorptive extraction (SBSE) and thermal desorption GC/MSD/PFPD. In Proceedings of the 23rd International Symposium on Capillary Chromatography, Riva del Garda, Italy, 5–10 June 2000; pp. 5–10.
- Esteban, J.; Valero, E.; Miranda, E.; Jimenez, M.; Martinez-Castro, I.; Sanz, J.; Morales, R. Automatic thermal desorption in GC and GC-MS analysis of volatile food components using conventional and chiral capillary columns. LC GC 1997, 15, 264–275.
- Prieto, A.; Basauri, O.; Rodil, R.; Usobiaga, A.; Fernández, L.; Etxebarria, N.; Zuloaga, O. Stir-bar sorptive extraction: A view on method optimisation, novel applications, limitations and potential solutions. J. Chromatogr. A 2010, 1217, 2642–2666.
- Ochiai, N.; Sasamoto, K.; David, F.; Sandra, P. Solvent-assisted stir bar sorptive extraction by using swollen polydimethylsiloxane for enhanced recovery of polar solutes in aqueous samples: Application to aroma compounds in beer and pesticides in wine. J. Chromatogr. A 2016, 1455, 45–56.
- Schiano, A.; Benoist, D.; Drake, M. Comparison of 3 rapid methods for analysis of vitamin degradation compounds in fluid skim milk. J. Dairy Sci. 2019, 102, 4906–4912.
- Qualley, A.V.; Dudareva, N. Metabolomics of plant volatiles. In Plant Systems Biology; Springer: Berlin/Heidelberg, Germany, 2009; pp. 329–343.
- Heaven, M.W.; Nash, D. Recent analyses using solid phase microextraction in industries related to food made into or from liquids. Food Control 2012, 27, 214–227.
- Vazquez-Landaverde, P.A.; Velazquez, G.; Torres, J.; Qian, M. Quantitative determination of thermally derived off-flavor compounds in milk using solid-phase microextraction and gas chromatography. J. Dairy Sci. 2005, 88, 3764–3772.
- Clarke, H.J.; Mannion, D.T.; O’Sullivan, M.G.; Kerry, J.P.; Kilcawley, K.N. Development of a headspace solid-phase microextraction gas chromatography mass spectrometry method for the quantification of volatiles associated with lipid oxidation in whole milk powder using response surface methodology. Food Chem. 2019, 292, 75–80.
- Wang, Z.; Zeng, G.; Wei, X.; Ding, B.; Huang, C.; Xu, B. Determination of vanillin and ethyl-vanillin in milk powder by headspace solid-phase microextraction coupled with gas chromatography-mass spectrometry. Food Anal. Methods 2016, 9, 3360–3366.
- García-Llatas, G.; Lagarda, M.J.; Romero, F.; Abellán, P.; Farré, R. A headspace solid-phase microextraction method of use in monitoring hexanal and pentane during storage: Application to liquid infant foods and powdered infant formulas. Food Chem. 2007, 101, 1078–1086.
- Jochmann, M.A.; Yuan, X.; Schilling, B.; Schmidt, T.C. In-tube extraction for enrichment of volatile organic hydrocarbons from aqueous samples. J. Chromatogr. A 2008, 1179, 96–105.
- Bendall, J.G. Aroma compounds of fresh milk from New Zealand cows fed different diets. J. Agric. Food Chem. 2001, 49, 4825–4832.
- Park, C.W.; Drake, M. Condensed milk storage and evaporation affect the flavor of nonfat dry milk. J. Dairy Sci. 2016, 99, 9586–9597.
- Bader, N. Stir bar sorptive extraction as a sample preparation technique for chromatographic analysis: An overview. Asian J. Nanosci. Mater. 2018, 1, 54–60.
- Markes International. Flavour Profiling of Milk Using HiSorb Sorptive Extraction and TD–GC–MS. Application Note 120. 2016. Available online: https://kinesis-australia.com.au/media/wysiwyg/knowledebase/pdf/Flavour_profiling_of_various_drinks_using_HiSorb_sorptive_extraction_and_TD_GC_MS.pdf (accessed on 28 September 2021).
- Clarke, H.J.; O’Sullivan, M.G.; Kerry, J.P.; Kilcawley, K.N. Correlating Volatile Lipid Oxidation Compounds with Consumer Sensory Data in Dairy Based Powders during Storage. Antioxidants 2020, 9, 338.
- Clarke, H.J.; Griffin, C.; Rai, D.K.; O’Callaghan, T.F.; O’Sullivan, M.G.; Kerry, J.P.; Kilcawley, K.N. Dietary Compounds Influencing the Sensorial, Volatile and Phytochemical Properties of Bovine Milk. Molecules 2020, 25, 26.
- Toso, B.; Procida, G.; Stefanon, B. Determination of volatile compounds in cows’ milk using headspace GC-MS. J. Dairy Res. 2002, 69, 569–577.
- Mannion, D.T.; Furey, A.; Kilcawley, K.N. Comparison and validation of 2 analytical methods for the determination of free fatty acids in dairy products by gas chromatography with flame ionization detection. J. Dairy Sci. 2016, 99, 5047–5063.
- Karagül-Yüceer, Y.; Cadwallader, K.R.; Drake, M. Volatile flavor components of stored nonfat dry milk. J. Agric. Food Chem. 2002, 50, 305–312.
- Tranchida, P.Q.; Salivo, S.; Bonaccorsi, I.; Rotondo, A.; Dugo, P.; Mondello, L. Analysis of the unsaponifiable fraction of lipids belonging to various milk-types by using comprehensive two-dimensional gas chromatography with dual mass spectrometry/flame ionization detection and with the support of high resolution time-of-flight mass spectrometry for structural elucidation. J. Chromatogr. A 2013, 1313, 194–201.
