Mevalonate Kinase Deficiency (MKD) is a rare inborn disease belonging to the family of periodic fever syndromes. The MKD phenotype is characterized by systemic inflammation involving multiple organs, including the nervous system. Current anti-inflammatory approaches to MKD are only partially effective and do not act specifically on neural inflammation. According to the new emerging pharmacology trends, the repositioning of drugs from the indication for which they were originally intended to another one can make mechanistic-based medications easily available to treat rare diseases. According to this perspective, the squalene synthase inhibitor Lapaquistat (TAK-475), originally developed as a cholesterol-lowering drug, might find a new indication in MKD, by modulating the mevalonate cholesterol pathway, increasing the availability of anti-inflammatory isoprenoid intermediates.
- mevalonate
- inflammation
- drug repositioning
- rare disease
[1][2][3][4][5][6][7][8][9][10][11][12][13][14][15][16][17][18][19][20][21][22][23][24][25][26][27][28][29][30][31][32][33][34][35]1. Introduction
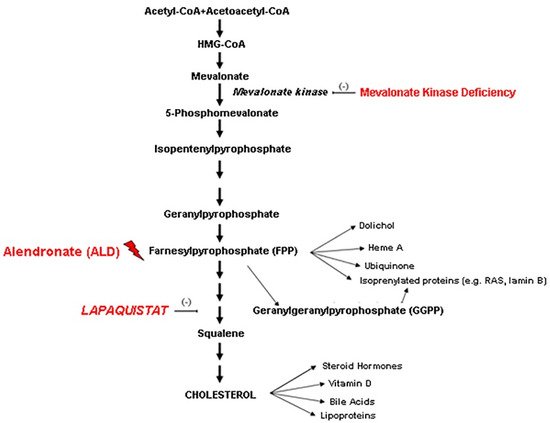
2. Discussion
3. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/biom11101438
References
- Irina Buhaescu; Hassane Izzedine; Mevalonate pathway: A review of clinical and therapeutical implications. Clinical Biochemistry 2007, 40, 575-584, 10.1016/j.clinbiochem.2007.03.016.
- Joost P.H. Drenth; Laurence Cuisset; Gilles Grateau; Christian Vasseur; Saskia D. Van De Velde-Visser; Jan G.N. De Jong; Jacques S Beckmann; Jos W.M. Van Der Meer; Marc Delpech & Contributing Members Of T; Mutations in the gene encoding mevalonate kinase cause hyper-IgD and periodic fever syndrome. Nature Genetics 1999, 22, 178-181, 10.1038/9696.
- Sander M Houten; Christiaan S van Woerden; Frits A Wijburg; Ronald J A Wanders; Hans R Waterham; Carrier frequency of the V377I (1129G>A) MVK mutation, associated with Hyper-IgD and periodic fever syndrome, in the Netherlands. European Journal of Human Genetics 2003, 11, 196-200, 10.1038/sj.ejhg.5200933.
- Georg Hoffmann; Kenneth M. Gibson; Ira K. Brandt; Patricia I. Bader; Rebecca S. Wappner; Lawrence Sweetman; Mevalonic Aciduria — An Inborn Error of Cholesterol and Nonsterol Isoprene Biosynthesis. New England Journal of Medicine 1986, 314, 1610-1614, 10.1056/nejm198606193142504.
- Naga Venkata Muralikrishna Akula; Man Shi; Zhaozhao Jiang; Celia E. Foster; David Miao; Annie S. Li; Xiaoman Zhang; Ruth M. Gavin; Sorcha D. Forde; Gail Germain; et al. Control of the innate immune response by the mevalonate pathway. Nature Immunology 2016, 17, 922-929, 10.1038/ni.3487.
- Yong Hwan Park; Geryl Wood; Daniel L. Kastner; Jae Jin Chae; Pyrin inflammasome activation and RhoA signaling in the autoinflammatory diseases FMF and HIDS. Nature Immunology 2016, 17, 914-921, 10.1038/ni.3457.
- José Noel Ibrahim; Isabelle Jéru; Jean-Claude Lecron; Myrna Medlej-Hashim; Cytokine signatures in hereditary fever syndromes (HFS). Cytokine & Growth Factor Reviews 2016, 33, 19-34, 10.1016/j.cytogfr.2016.11.001.
- Annalisa Marcuzzi; Valentina Zanin; Giulio Kleiner; Lorenzo Monasta; Sergio Crovella; Mouse model of mevalonate kinase deficiency: comparison of cytokine and chemokine profile with that of human patients. Pediatric Research 2013, 74, 266-271, 10.1038/pr.2013.96.
- Annalisa Marcuzzi; Elisa Piscianz; Liza Vecchi Brumatti; Alberto Tommasini; Mevalonate kinase deficiency: therapeutic targets, treatments, and outcomes. Expert Opinion on Orphan Drugs 2017, 5, 515-524, 10.1080/21678707.2017.1328308.
- Annalisa Marcuzzi; Claudia Loganes; Erica Valencic; Elisa Piscianz; Lorenzo Monasta; Sabrine Bilel; Roberta Bortul; Claudio Celeghini; Marina Zweyer; Alberto Tommasini; et al. Neuronal Dysfunction Associated with Cholesterol Deregulation. International Journal of Molecular Sciences 2018, 19, 1523, 10.3390/ijms19051523.
- Fabrizio De Benedetti; Marco Gattorno; Jordi Anton; Eldad Ben-Chetrit; Joost Frenkel; Hal M. Hoffman; Isabelle Koné-Paut; Helen Lachmann; Seza Ozen; Anna Simon; et al. Canakinumab for the Treatment of Autoinflammatory Recurrent Fever Syndromes. New England Journal of Medicine 2018, 378, 1908-1919, 10.1056/nejmoa1706314.
- Hana Malcova; Zuzana Strizova; Tomas Milota; Ilja Striz; Anna Sediva; Dita Cebecauerova; Rudolf Horvath; IL-1 Inhibitors in the Treatment of Monogenic Periodic Fever Syndromes: From the Past to the Future Perspectives. Frontiers in Immunology 2021, 11, 619257, 10.3389/fimmu.2020.619257.
- Marit S. Schneiders; Sander M. Houten; Marjolein Turkenburg; Ronald J. A. Wanders; Hans R. Waterham; Manipulation of isoprenoid biosynthesis as a possible therapeutic option in mevalonate kinase deficiency. Arthritis & Rheumatism 2006, 54, 2306-2313, 10.1002/art.21960.
- Tomoyuki Nishimoto; Yuichiro Amano; Ryuichi Tozawa; Eiichiro Ishikawa; Yoshimi Imura; Hidefumi Yukimasa; Yasuo Sugiyama; Lipid-lowering properties of TAK-475, a squalene synthase inhibitor,in vivoandin vitro. Journal of Cerebral Blood Flow & Metabolism 2003, 139, 911-918, 10.1038/sj.bjp.0705332.
- Yuichiro Amano; Tomoyuki Nishimoto; Ryu Ichi Tozawa; Eiichiro Ishikawa; Yoshimi Imura; Yasuo Sugiyama; Lipid-lowering effects of TAK-475, a squalene synthase inhibitor, in animal models of familial hypercholesterolemia.. European Journal of Pharmacology 2003, 466, 155-161, 10.1016/s0014-2999(03)01549-8.
- Linda Henneman; Arno G. van Cruchten; Willem Kulik; Hans R. Waterham; Inhibition of the isoprenoid biosynthesis pathway; detection of intermediates by UPLC–MS/MS. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids 2011, 1811, 227-233, 10.1016/j.bbalip.2011.01.002.
- Yoshihiro Shidoji; Yuki Tabata; Unequivocal evidence for endogenous geranylgeranoic acid biosynthesized from mevalonate in mammalian cells. Journal of Lipid Research 2019, 60, 579-593, 10.1194/jlr.m090548.
- Annalisa Marcuzzi; Elisa Piscianz; Marina Zweyer; Roberta Bortul; Claudia Loganes; Martina Girardelli; Gabriele Baj; Lorenzo Monasta; Claudio Celeghini; Geranylgeraniol and Neurological Impairment: Involvement of Apoptosis and Mitochondrial Morphology. International Journal of Molecular Sciences 2016, 17, 365, 10.3390/ijms17030365.
- Annalisa Marcuzzi; Claudia Loganes; Claudio Celeghini; Giulio Kleiner; Repositioning of Tak-475 In Mevalonate Kinase Disease: Translating Theory Into Practice. Current Medicinal Chemistry 2018, 25, 2783-2796, 10.2174/0929867324666170911161417.
- T. Ebihara; K. Teshima; T. Kondo; Y. Tagawa; T. Moriwaki; S. Asahi; Pharmacokinetics of TAK-475, a Squalene Synthase Inhibitor, in Rats and Dogs. Drug Research 2016, 66, 287-292, 10.1055/s-0035-1569407.
- Stephanie Seiki; William H. Frishman; Pharmacologic Inhibition of Squalene Synthase and Other Downstream Enzymes of the Cholesterol Synthesis Pathway. Cardiology in Review 2009, 17, 70-76, 10.1097/crd.0b013e3181885905.
- James K. Liao; Squalene synthase inhibitor lapaquistat acetate: could anything be better than statins?. Circulation 2011, 123, 1925-1928, 10.1161/CIRCULATIONAHA.111.028571.
- Eeva-Liisa Eskelinen; To be or not to be? Examples of incorrect identification of autophagic compartments in conventional transmission electron microscopy of mammalian cells. Autophagy 2008, 4, 257-260, 10.4161/auto.5179.
- Aviva M. Tolkovsky; Mitophagy. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research 2009, 1793, 1508-1515, 10.1016/j.bbamcr.2009.03.002.
- Davide Martorana; Francesco Bonatti; Paola Mozzoni; Augusto Vaglio; Antonio Percesepe; Monogenic Autoinflammatory Diseases with Mendelian Inheritance: Genes, Mutations, and Genotype/Phenotype Correlations. Frontiers in Immunology 2017, 8, 344, 10.3389/fimmu.2017.00344.
- Jerold Jeyaratnam; Joost Frenkel; Management of Mevalonate Kinase Deficiency: A Pediatric Perspective. Frontiers in Immunology 2020, 11, 1150, 10.3389/fimmu.2020.01150.
- Giuseppe Filomeni; Daniela De Zio; Francesco Cecconi; Oxidative stress and autophagy: the clash between damage and metabolic needs. Cell Death & Differentiation 2014, 22, 377-388, 10.1038/cdd.2014.150.
- Meng Bao; Zhengjun Yi; Yurong Fu; Activation of TLR7 Inhibition of Mycobacterium Tuberculosis Survival by Autophagy in RAW 264.7 Macrophages. Journal of Cellular Biochemistry 2017, 118, 4222-4229, 10.1002/jcb.26072.
- Anna Simon; Elizabeth Drewe; Jos W. M. Van Der Meer Md; Richard J. Powell; Richard I. Kelley Md; Anton F. H. Stalenhoef Md; Joost P. H. Drenth Md; Simvastatin treatment for inflammatory attacks of the hyperimmunoglobulinemia D and periodic fever syndrome. Clinical Pharmacology & Therapeutics 2004, 75, 476-483, 10.1016/j.clpt.2004.01.012.
- G F Hoffmann; C Charpentier; E Mayatepek; J Mancini; M Leichsenring; K M Gibson; P Divry; Martin Hrebicek; W Lehnert; K Sartor; et al. Clinical and biochemical phenotype in 11 patients with mevalonic aciduria.. Pediatrics 1993, 91, 915-21, .
- Christoph Hübner; Georg F Hoffmann; Christiane Charpentier; Kenneth M Gibson; Barbara Finckh; Herbert Puhl; Hans-Anton Lehr; Alfred Kohlschütter; Decreased Plasma Ubiquinone-10 Concentration in Patients with Mevalonate Kinase Deficiency. Pediatric Research 1993, 34, 129-133, 10.1203/00006450-199308000-00004.
- Brett N. Olsen; Paul H. Schlesinger; Daniel S. Ory; Nathan A. Baker; 25-Hydroxycholesterol Increases the Availability of Cholesterol in Phospholipid Membranes. Biophysical Journal 2011, 100, 948-956, 10.1016/j.bpj.2010.12.3728.
- Josef Finsterer; Repurposed Drugs in Metabolic Disorders. Current Topics in Medicinal Chemistry 2013, 13, 2386-2394, 10.2174/15680266113136660166.
- Maryam Lotfi Shahreza; Nasser Ghadiri; Sayed Rasoul Mousavi; Jaleh Varshosaz; James Green; A review of network-based approaches to drug repositioning. Briefings in Bioinformatics 2017, 19, 878-892, 10.1093/bib/bbx017.
- Nobutaka Suzuki; Tatsuo Ito; Hisanori Matsui; Masayuki Takizawa; Anti-inflammatory and cytoprotective effects of a squalene synthase inhibitor, TAK-475 active metabolite-I, in immune cells simulating mevalonate kinase deficiency (MKD)-like condition. SpringerPlus 2016, 5, 1429-1429, 10.1186/s40064-016-3125-1.
