Molecular targeted therapy was reported to have fewer adverse effects and offer a more convenient route of administration compared with conventional chemotherapy. With the development of sequencing technology and research on the molecular biology of lung cancer, especially whole-genome information on non-small-cell lung cancer (NSCLC), various therapeutic targets have been unveiled. Among the NSCLC-driving gene mutations, epidermal growth factor receptor (EGFR) mutations are the most common driver gene, and approximately 10% of Caucasian and more than 50% of Asian NSCLC patients have been found to have sensitive EGFR mutations. A variety of targeted therapeutic agents for EGFR mutations have been approved for clinical applications or are undergoing clinical trials around the world. This review is focused on the indications of approved small molecular kinase inhibitors for EGFR mutation-positive NSCLC, the mechanisms of drug resistance and the corresponding therapeutic strategies, as well as the principle of reasonable and precision molecular structure for drug development discovery of next-generation inhibitors for EGFR, which would accelerate anticancer drug discovery.
- epidermal growth factor receptor tyrosine kinase inhibitors
- EGFR mutations
- molecular targeted therapy
- non-small cell lung cancer
- resistance mechanism
1. Introduction
Lung cancer is a serious threat to human health [1]. A global cancer report published by the World Health Organization (WHO) in 2020 reports that lung cancer is still the most common and fatal cancer, and it affects both men and women [2]. Lung cancer patients not only experience great psychological pressure, but also suffer severe pain due to the disease. Moreover, the high cost of treatment presents a great burden to both individuals and society [3].
The goal of advanced lung cancer treatment is to prolong the overall survival time of patients and to improve their quality of life [4]. Chemotherapy is one approach that kills cancer cells, and it can be administered orally or by injection. Platinum-based chemotherapy in combination with other cytotoxic drugs for 4–6 cycles is the current routine treatment. However, chemotherapy is not recommended for elderly patients with weak conditions, cachexia, serious dysfunction of the heart, liver, or kidney, or poor bone marrow function. With the advanced improvement of sequencing technology and in-depth research on the molecular biology of lung cancer, especially whole-genome sequencing, targeted therapy for solid tumors has rapidly developed. This has brought good news for, especially advanced, non-small-cell lung cancer (NSCLC) patients.
With the advancements in modern medicine, the diagnosis of, and therapy for, NSCLC have entered the era of “precision medicine”, facilitating more accurate diagnosis and treatment. According to the latest National Comprehensive Cancer Network (NCCN) guidelines for NSCLC (3rd Edition, 2021), the genes with driver mutations include: epidermal growth factor receptor ( EGFR ); anaplastic lymphoma kinase ( ALK ), c-ros oncogene 1 receptor tyrosine kinase ( ROS1 ); human epidermal growth factor receptor 2 (HER2); mesenchymal to epithelial transition factor (MET); v-raf murine sarcoma viral oncogene homolog B1 ( BRAF ); Kirsten rat sarcoma ( KRAS ); rearrangement during transfection ( RET ); and neurotrophic tyrosine receptor kinase ( NTRK ) [5]. Individualized molecular targeted therapy for driver genes has been reported to block the key signaling pathway of tumor cell growth and proliferation, inhibit tumor cell proliferation, and selectively kill tumor cells by applying highly specific molecules targeted to the definite (or highly expressed) biomarkers of the tumor cells [6]. In recent decades, a variety of targeted therapeutic agents have been approved for clinical applications, or are undergoing clinical trials, that have become the standard treatment for advanced lung cancer because of their significant efficacy and safety.
Among the driver mutations of NSCLC, EGFR mutation is the most conventional driver gene in NSCLC, and approximately 10% of Caucasian, and more than 50% of Asian, NSCLC patients have been found to have sensitive EGFR mutations [7]. EGFR is reported as a subtype of the erythroblastosis oncogene B (ErbB)/human epidermal growth factor receptor (HER) family, which is also named ErbB1 (EGFR/HER1) [8][9]. As a transmembrane tyrosine kinase receptor, activated EGFR was reported to facilitate signal transduction in critical pathways of tumorigenesis [10][11]. Since the 1990s, various drug development strategies for the inhibition of overactivated EGFR have been carried out. The EGFR-targeted drugs for NSCLC that are available on the market are classified into two major categories: EGFR monoclonal antibody drugs that block the binding of extracellular ligand receptors, and small molecule chemical kinase inhibitors that inhibit the intracellular ATP binding site of tyrosine kinase [12]. Currently, the approved EGFR monoclonal antibodies are nimotuzumab and necitumumab, which are applied in combination with chemotherapy drugs in the clinic [13][14][15]. Treatment with epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) is considered more convenient, and can significantly prolong the survival time of patients, compared with the expensive therapeutic regimen of antibodies. In this review, we will focus on the indications of approved inhibitors for EGFR mutation-positive advanced NSCLC, the mechanisms of drug resistance and the corresponding therapeutic strategies, as well as the principles of reasonable and precision molecular structure for the discovery of next-generation EGFR-TKIs in order to accelerate anticancer drug discovery.
2. The Research Progress of EGFR-TKIs in NSCLC
The discovery of EGFR mutations in 2004 changed the standard treatment of NSCLC and established a new treatment mode according to the new molecular typing. The incidence rate of EGFR mutations is higher among women and nonsmokers. Interestingly, EGFR mutations appear widely in Asian populations [16]. The Prospective Analysis of Oncogenic Driver Mutations and Environmental Factors (PIONEER) study analyzed the tumors of 1482 patients with adenocarcinoma in mainland China, Hong Kong, Taiwan, and four other countries in Asia, including India, the Philippines, Thailand, and Vietnam, and the incidence of EGFR mutations in advanced lung adenocarcinoma was 51.4% [17]. Although EGFR mutations are more common in women and nonsmokers, they are also present in men and in 37% of regular smokers.
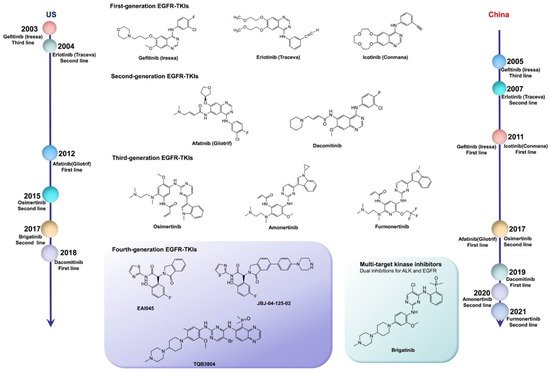
EGFR mutations are classified as: classical sensitive mutations (EGFR exon 19 deletion (ex19del) mutation and EGFR exon 21 p. L858R (L858R) mutation); EGFR nonclassical mutations (approximately 10%); and EGFR exon 20 insertion (ex20ins) mutations (approximately 7%) [18]. The IPASS study first proved that the first-line use of EGFR-TKIs in patients with EGFR-sensitive mutations can notably increase the objective response rate (ORR), and significantly prolong progression-free survival (PFS), compared with the results obtained from chemotherapy. Therefore, the first-line use of EGFR-TKIs has become the standard for advanced NSCLC patients with sensitizing EGFR mutations. The US Food and Drug Administration (FDA) has approved a series of EGFR-TKIs, including gefitinib, erlotinib, afatinib, dacomitinib, and osimertinib, as the first-line treatment of advanced lung cancer with EGFR mutations [19]. In China, icotinib, almonertinib, and alftinib, as original drugs, have been approved by the National Medical Products Administration (NMPA) for the treatment of NSCLC with sensitizing EGFR mutations. In addition, olmutinib was launched in South Korea as a second-line treatment for patients with advanced or metastatic NSCLC with EGFR exon 20 p. T790M (T790M) mutation positivity in 2016 ( Table 1 ) [20].
| Product Name | Phase | Company | Trial ID | Study Design | mPFS (Months) |
HR | mOS (Months) | ORR | DCR | Control Group | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gefitinib | III | AstraZeneca | NCT00322452 | RCT | 9.5 vs. 6.3 | 0.48 | 21.6 vs. 21.9 | 71.2% vs. 47.3% | / | Carboplatin + Paclitaxel | [21] |
| Icotinib | III | China | NCT01040780 | RCT | 4.5 vs. 3.4 | 0.84 | 13.3 vs. 13.9 | 27.6% vs. 27.2% | 75.4% vs. 74.9% | gefitinib | [22] |
| Icotinib | III | China | NCT01719536 | RCT | 11.2 vs. 6.9 | 0.61 | 30.5 vs. 32.1 | 64.8% vs. 33.85% | / | Cisplatin + pemetrexed | [23] |
| Erlotinib | III | Roche | NCT00446225 | RCT | 9.7 vs. 5.2 | 0.37 | 19.3 vs. 19.5 | 55% vs. 11% | 78% vs. 66% | platinum-containing dual drugs | [24] |
| Erlotinib | III | Roche | NCT00874419 | RCT | 13.7 vs. 4.6 | 0.16 | 22.8 vs. 27.2 | 83% vs. 36% | 96% vs. 82% | gemcitabine/carboplatin | [25] |
| Afatinib | III | Boehringer Ingelheim | NCT01121393 | RCT | 11.1 vs. 6.9 | 0.58 | 28.2 vs. 28.2 | 56% vs. 24% | 90% vs. 82% | cisplatin/pemetrexed | [26] |
| Afatinib | III | Boehringer Ingelheim | NCT01121393 | RCT | 11.0 vs. 5.6 | 0.28 | 23.1 vs. 23.2 | 66.9% vs. 23.0% | 92.6% vs. 76.2% | cisplatin/gemcitabine | [27] |
| Afatinib | II | Boehringer Ingelheim | NCT01466660 | RCT | 11 vs. 10.9 | 0.74 | 27.9 vs. 24.5 | 70% vs. 56% | 91.3% vs. 87.4% | gefitinib | [28] |
| Afatinib | III | Boehringer Ingelheim | NCT01523587 | RCT | 2.6 vs. 1.9 | 0.81 | 7.9 vs. 6.8 | 5.5% vs. 2.8% | 50.5% vs. 39.5% | erlotinib | [29] |
| Dacomitinib | III | Pfizer | NCT00446225 | RCT | 14.7 vs. 9.2 | 0.59 | 34.1 vs. 26.8 | 74.9% vs. 71.6% | / | gefitinib | [30] |
| Gefitinib | III | AstraZeneca | WJTOG3405 | RCT | 9.2 vs. 6.3 | 0.49 | 34.9 vs. 37.3 | 62.1% vs. 32.2% | 93.1% vs. 78% | cisplatin/docetaxel | [31] |
| Gefitinib | III | AstraZeneca | NEJ0020376 | RCT | 10.8 vs. 5.4 | 0.32 | 27.7 vs. 26.6 | 73.7% vs. 30.7% | / | carboplatin/paclitaxel | [32] |
| Erlotinib | III | Roche | NCT01342965 | RCT | 11.0 vs. 5.5 | 0.34 | 26.3 vs. 25.5 | 62.7% vs. 33.6% | 89.1% vs. 76.6% | cisplatin/gemcitabine | [33] |
| Osimertinib | III | AstraZeneca | NCT02296125 | RCT | 18.9 vs. 10.2 | 0.42 | 38.6 vs. 31.8 | 80% vs. 76% | 97% vs. 92% | gefitinib/erlotinib | [34][35] |
| Osimertinib | III | AstraZeneca | NCT02151981 | RCT | 11.7 vs. 5.6 | 0.32 | / | 70% vs. 31% | 93% vs. 63% | platinum + pemetrexed | [36] |
| Osimertinib | II | AstraZeneca | NCT03424759 | Single arm studies | 8.2 | / | / | 50% | 89% | / | [37] |
| Osimertinib | II | AstraZeneca | NCT02228369 | Single arm studies | 8.6 | / | 11 | 41% | / | / | [38] |
| Almonertinib | II | China | NCT02981108 | Single arm studies | 12.3 | / | / | 68.9% | 93.4% | / | [39] |
| Olmutinib | II | South Korea | NCT02485652 | Single arm studies | 9.4 | / | 19.7 | 51.9% | 81.4% | / | [40] |
| Furmonertinib | II | China | NCT03452592 | Single arm studies | 9.6 | / | / | 74.1% | 93.6% | / | [41] |
RCT: randomized controlled trial; mPES: median progression-free survival; HR: hazard ratio; mOS: median overall survival; ORR: objective response rate; DCR: disease control rate.
Compared with the first-generation reversible TKIs on the market, second-generation irreversible EGFR inhibitors possess more binding sites, but result in dose-related adverse effects. Despite the significant therapeutic effects of individualized therapy, rather than traditional chemotherapy, most NSCLC patients present drug resistance after 1–2 years of treatment with first- or second-generation TKIs. More than 50% of the resistance mechanism was related to the T790M mutation. Moreover, the application of second-generation TKIs could not defeat the resistance to first-generation agents. For this reason, third-generation TKIs were designed with the superiority of binding to EGFR-sensitive mutations and T790M mutation sites, subsequently inhibiting the tumor resistance caused by the T790M mutation. However, osimeritinib, as a third-generation EGFR inhibitor, could not break the curse of drug resistance. Although the underlying mechanisms are complicated, the results of several reported clinical trials demonstrate that drug resistance still occurs. Inhibitors for different mutation targets are emerging, and various approaches could address resistance to osimeritinib. However, it is possible to overcome the obstacle of undetected mutation targets with the development of second-generation sequencing technology.
Fourth-generation EGFR-TKIs are expected to target EGFR exon 20 p. C797S (C797S) triple mutations after resistance to third-generation inhibitors; however, none of them have been officially approved to date. In 2016, compound EAI045 was reported by Nature to have effectively overcome, for the first time, the C797S mutation when combined with a monoclonal antibody. JBJ-04-125-02, which was further improved from the skeleton of EAI045, can effectively solve the problem of the C797S cis mutation. In addition, TQB3804, an original candidate in China that first appeared at the American Association for Cancer Research (AACR) conference in 2019, was developed to prevent EGFR C797S cis mutations ( Figure 1 ).
3. Conclusion and perspectives
With the development of biomedicine, patients with advanced NSCLC truly benefit from precision medicine. Most patients can choose targeted therapy with few adverse effects as the first-line therapeutic regimens. Compared with standard traditional chemotherapy, EGFR-TKIs as the first-line treatment for sensitive EGFR mutations can prolong PFS, improve quality of life and reduce severe adverse effects related to therapeutics, and they have become the primary treatment option for patients with advanced NSCLC. In recent decades, first-, second-, and third-generation EGFR-TKIs have been launched on the market with widespread clinical applications. Meanwhile, basic research and clinical trials of fourth-generation TKIs are also in progress. However, the occurrence and development of tumors refer to complex genetic mutations and signaling pathways, and the drug resistance of molecular targeted agents seems to be inevitable.
As a clinical therapeutic regimen for EGFR-mutant NSCLC, third-generation osimertinib has been proven to inhibit the novel T790M mutation of first- and second-generation TKIs. Fourth-generation compounds were initially designed to combat C797S mutation-mediated resistance to osimertinib. However, the inhibitory efficacy of osimertinib on L858R was significantly reduced after C797S mutation. Hence, the structure-based drug design of fourth-generation drugs also takes into account two common triple mutations. Over time, rare resistance mechanisms will be gradually unveiled. If the current research on drug discovery relies on updating the structural design of molecules on the basis of ascertainable mutations, the treatment cost for patients in the future would be beyond the acceptable budget, which is not in alignment with the original intention for drug discovery. Currently, the combination of osimertinib with other kinase inhibitors or antiangiogenics has been considered a promising therapeutic regimen in the clinic for acquired resistance. It is foreseeable that varying mechanisms of resistance to osimertinib may arise after combined application with other anticancer drugs, and whether the incidence of C797S mutation is affected following combination therapies remains to be determined. These are vital problems demanding prompt solutions in the discovery of next-generation targeted agents and the management of individualized therapies based on precision medicine.
The emergence of drug resistance is a gradual process. Currently, the detection of mutated EGFR genes for acquired resistance is conducted by tissue or blood biopsy after disease progression, and the last-line treatment option is consequently considered. PET/CT imaging technology based on targeted molecular probes has been developed and applied in preclinical research to conveniently monitor mutated genes during therapy in a timely manner 42. This innovative technology could provide diagnostic data and evidence for individualized clinical therapeutic regimens. However, the design of precise selective and sensitive targeted molecular probes is the key technology for the prediction of genetic mutations, which is extremely reliant upon comprehensive research and screening for structure-activity relationships 43.
With recent translational research on the biological mechanism of NSCLC progression, new pharmacotherapy approaches for protein kinase mutants continue to be developed. In addition to protein kinase inhibitors and monoclonal antibodies, targeted protein degradation is an emerging therapeutic strategy in anticancer drug discovery. In June 2021, C4 Therapeutics reported a protein degradation agent targeting EFGR mutation at the Virtual Meeting. CFT8919, as a mutant selective degrader, was developed to target the degraded EGFR L858R mutation. Meanwhile, CFT8919 was reported to be active against resistant mutations, such as EGFR T790M and C797S, but with low activity against EGFRWT, which indicated its potential clinical value for NSCLC patients.
Targeted therapy for lung cancer is a creative strategy with an exciting perspective that benefits more than half of patients. Future research trends and strategy processes for targeted therapy of NSCLC require novel drug development with high efficiency to overcome drug resistance, combined therapeutic options to benefit the long-term survival of NSCLC patients, clinical therapeutic regimens grounded on the characteristics and genotypes of patients, and individualized whole process management schemes on the basis of precision medicine. In summary, with the development of drug discovery and the innovation of therapeutic strategies in the future, lung cancer is expected to be a curable chronic disease.
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics13091500
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424.
- Wild, C.; Weiderpass, E.; Stewart, B.W. World Cancer Report: Cancer Research for Cancer Prevention; IARC Press: Lyon, France, 2020.
- Herbst, R.S.; Morgensztern, D.; Boshoff, C. The biology and management of non-small cell lung cancer. Nature 2018, 553, 446–454.
- Yu, L.; Chen, Y.; Tooze, S.A. Autophagy pathway: Cellular and molecular mechanisms. Autophagy 2018, 14, 207–215.
- Ettinger, D.S.; Wood, D.E.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R.; D’Amico, T.A. NCCN Guidelines Insights: Non–Small Cell Lung Cancer, Version 2.2021: Featured Updates to the NCCN Guidelines. J. Natl. Compr. Cancer Netw. 2021, 19, 254–266.
- Kris, M.G.; Johnson, B.E.; Berry, L.D.; Kwiatkowski, D.J.; Iafrate, A.J.; Wistuba, I.I.; Varella-Garcia, M.; Franklin, W.A.; Aronson, S.L.; Su, P.-F. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA 2014, 311, 1998–2006.
- Zhang, J.; Zhao, X.; Zhao, Y.; Zhang, J.; Zhang, Z.; Wang, J.; Wang, Y.; Dai, M.; Han, J. Value of pre-therapy 18 F-FDG PET/CT radiomics in predicting EGFR mutation status in patients with non-small cell lung cancer. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1137–1146.
- Arteaga, C.L.; Engelman, J.A. ERBB receptors: From oncogene discovery to basic science to mechanism-based cancer therapeutics. Cancer Cell 2014, 25, 282–303.
- Tebbutt, N.; Pedersen, M.W.; Johns, T.G. Targeting the ERBB family in cancer: Couples therapy. Nat. Rev. Cancer 2013, 13, 663–673.
- Roskoski, R., Jr. Small molecule inhibitors targeting the EGFR/ErbB family of protein-tyrosine kinases in human cancers. Pharmacol. Res. 2019, 139, 395–411.
- Vecchione, L.; Jacobs, B.; Normanno, N.; Ciardiello, F.; Tejpar, S. EGFR-targeted therapy. Exp. Cell Res. 2011, 317, 2765–2771.
- Yin, Y.; Yuan, X.; Gao, H.; Yang, Q. Nanoformulations of small molecule protein tyrosine kinases inhibitors potentiate targeted cancer therapy. Int. J. Pharm. 2020, 573, 118785.
- Talavera, A.; Friemann, R.; Gómez-Puerta, S.; Martinez-Fleites, C.; Garrido, G.; Rabasa, A.; López-Requena, A.; Pupo, A.; Johansen, R.F.; Sánchez, O. Nimotuzumab, an antitumor antibody that targets the epidermal growth factor receptor, blocks ligand binding while permitting the active receptor conformation. Cancer Res. 2009, 69, 5851–5859.
- Díaz-Serrano, A.; Sánchez-Torre, A.; Paz-Ares, L. Necitumumab for the treatment of advanced non-small-cell lung cancer. Future Oncol. 2019, 15, 705–716.
- Cai, W.-Q.; Zeng, L.-S.; Wang, L.-F.; Wang, Y.-Y.; Cheng, J.-T.; Zhang, Y.; Han, Z.-W.; Zhou, Y.; Huang, S.-L.; Wang, X.-W. The latest battles between EGFR monoclonal antibodies and resistant tumor cells. Front. Oncol. 2020, 10, 1249.
- Kawaguchi, T.; Koh, Y.; Ando, M.; Ito, N.; Takeo, S.; Adachi, H.; Tagawa, T.; Kakegawa, S.; Yamashita, M.; Kataoka, K. Prospective analysis of oncogenic driver mutations and environmental factors: Japan molecular epidemiology for lung cancer study. J. Clinic. Oncol. 2016, 34, 2247–2257.
- Shi, Y.; Au, J.S.-K.; Thongprasert, S.; Srinivasan, S.; Tsai, C.-M.; Khoa, M.T.; Heeroma, K.; Itoh, Y.; Cornelio, G.; Yang, P.-C. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non–small-cell lung cancer of adenocarcinoma histology (PIONEER). J. Thorac. Oncol. 2014, 9, 154–162.
- Fukuoka, M.; Wu, Y.-L.; Thongprasert, S.; Sunpaweravong, P.; Leong, S.-S.; Sriuranpong, V.; Chao, T.-Y.; Nakagawa, K.; Chu, D.-T.; Saijo, N. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non–small-cell lung cancer in Asia (IPASS). J. Clinic. Oncol. 2011, 29, 2866–2874.
- Zhang, H.F.; Ma, J.S.; Li, L.; Gao, Q.; Wang, T. Comparative efficacy and safety of first-line EGFR-TKIs for advanced non-small cell lung cancer: A network meta-analysis. Chin. J. Dis. Control Prevn. 2020, 24, 210–216.
- Grigoriu, B.; Berghmans, T.; Meert, A.-P. Management of EGFR mutated nonsmall cell lung carcinoma patients. Eur. Respir. J. 2015, 45, 1132–1141.
- Shepherd, F.A. Molecular selection trumps clinical selection. J. Clinic. Oncol. 2011, 29, 2843–2844.
- Shi, Y.; Zhang, L.; Liu, X.; Zhou, C.; Zhang, S.; Wang, D.; Li, Q.; Qin, S.; Hu, C.; Zhang, Y. Icotinib versus gefitinib in previously treated advanced non-small-cell lung cancer (ICOGEN): A randomised, double-blind phase 3 non-inferiority trial. Lancet Oncol. 2013, 14, 953–961.
- Shi, Y.K.; Wang, L.; Han, B.e.; Li, W.; Yu, P.; Liu, Y.; Ding, C.; Song, X.; Ma, Z.; Ren, X. First-line icotinib versus cisplatin/pemetrexed plus pemetrexed maintenance therapy for patients with advancedEGFR mutation-positive lung adenocarcinoma (CONVINCE): A phase 3, open-label, randomized study. Ann. Oncol. 2017, 28, 2443–2450.
- Rosell, R.; Carcereny, E.; Gervais, R.; Vergnenegre, A.; Massuti, B.; Felip, E.; Palmero, R.; Garcia-Gomez, R.; Pallares, C.; Sanchez, J.M. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012, 13, 239–246.
- Zhou, C.; Wu, Y.-L.; Chen, G.; Feng, J.; Liu, X.-Q.; Wang, C.; Zhang, S.; Wang, J.; Zhou, S.; Ren, S. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): A multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011, 12, 735–742.
- Sequist, L.V.; Yang, J.C.H.; Yamamoto, N.; O’Byrne, K.; Hirsh, V.; Mok, T.; Geater, S.L.; Orlov, S.; Tsai, C.M.; Boyer, M. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J. Clinic. Oncol. 2013, 31, 3327–3334.
- Wu, Y.-L.; Zhou, C.; Hu, C.-P.; Feng, J.; Lu, S.; Huang, Y.; Li, W.; Hou, M.; Shi, J.H.; Lee, K.Y. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): An open-label, randomised phase 3 trial. Lancet Oncol. 2014, 15, 213–222.
- Park, K.; Tan, E.-H.; O’Byrne, K.; Zhang, L.; Boyer, M.; Mok, T.; Hirsh, V.; Yang, J.C.-H.; Lee, K.H.; Lu, S. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): A phase 2B, open-label, randomised controlled trial. Lancet Oncol. 2016, 17, 577–589.
- Soria, J.-C.; Felip, E.; Cobo, M.; Lu, S.; Syrigos, K.; Lee, K.H.; Göker, E.; Georgoulias, V.; Li, W.; Isla, D. Afatinib versus erlotinib as second-line treatment of patients with advanced squamous cell carcinoma of the lung (LUX-Lung 8): An open-label randomised controlled phase 3 trial. Lancet Oncol. 2015, 16, 897–907.
- Wu, Y.-L.; Cheng, Y.; Zhou, X.; Lee, K.H.; Nakagawa, K.; Niho, S.; Tsuji, F.; Linke, R.; Rosell, R.; Corral, J. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): A randomised, open-label, phase 3 trial. Lancet Oncol. 2017, 18, 1454–1466.
- Mitsudomi, T.; Morita, S.; Yatabe, Y.; Negoro, S.; Okamoto, I.; Tsurutani, J.; Seto, T.; Satouchi, M.; Tada, H.; Hirashima, T. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): An open label, randomised phase 3 trial. Lancet Oncol. 2010, 11, 121–128.
- Inoue, A.; Kobayashi, K.; Maemondo, M.; Sugawara, S.; Oizumi, S.; Isobe, H.; Gemma, A.; Harada, M.; Yoshizawa, H.; Kinoshita, I. Updated overall survival results from a randomized phase III trial comparing gefitinib with carboplatin–paclitaxel for chemo-naïve non-small cell lung cancer with sensitive EGFR gene mutations (NEJ002). Ann. Oncol. 2013, 24, 54–59.
- Wu, Y.-L.; Zhou, C.; Liam, C.-K.; Wu, G.; Liu, X.; Zhong, Z.; Lu, S.; Cheng, Y.; Han, B.; Chen, L. First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small-cell lung cancer: Analyses from the phase III, randomized, open-label, ENSURE study. Ann. Oncol. 2015, 26, 1883–1889.
- Soria, J.-C.; Ohe, Y.; Vansteenkiste, J.; Reungwetwattana, T.; Chewaskulyong, B.; Lee, K.H.; Dechaphunkul, A.; Imamura, F.; Nogami, N.; Kurata, T. Osimertinib in untreated EGFR-mutated advanced non–small-cell lung cancer. N. Engl. J. Med. 2018, 378, 113–125.
- Ramalingam, S.S.; Vansteenkiste, J.; Planchard, D.; Cho, B.C.; Gray, J.E.; Ohe, Y.; Zhou, C.; Reungwetwattana, T.; Cheng, Y.; Chewaskulyong, B. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N. Engl. J. Med. 2020, 382, 41–50.
- Wu, Y.-L.; Ahn, M.-J.; Garassino, M.C.; Han, J.-Y.; Katakami, N.; Kim, H.R.; Hodge, R.; Kaur, P.; Brown, A.P.; Ghiorghiu, D. CNS efficacy of osimertinib in patients with T790M-positive advanced non–small-cell lung cancer: Data from a randomized phase III trial (AURA3). J. Clin. Oncol. 2018, 36, 2702–2709.
- Cho, J.H.; Lim, S.H.; An, H.J.; Kim, K.H.; Park, K.U.; Kang, E.J.; Choi, Y.H.; Ahn, M.S.; Lee, M.H.; Sun, J.-M. Osimertinib for patients with non–small-cell lung cancer harboring uncommon EGFR mutations: A multicenter, open-label, phase II trial (KCSG-LU15-09). J. Clin. Oncol. 2020, 38, 488.
- Yang, J.C.; Kim, S.-W.; Kim, D.-W.; Lee, J.-S.; Cho, B.C.; Ahn, J.-S.; Lee, D.H.; Kim, T.M.; Goldman, J.W.; Natale, R.B. Osimertinib in patients with epidermal growth factor receptor mutation–positive non–small-cell lung cancer and leptomeningeal metastases: The BLOOM study. J. Clin. Oncol. 2020, 38, 538.
- Lu, S.; Wang, Q.; Zhang, G. A multicenter, open-label, single-arm, phase II study: The third generation EGFR tyrosine kinase inhibitor almonertinib for pretreated EGFR T790M-positive locally advanced or metastatic non-small cell lung cancer (APOLLO). In Proceedings of the AACR Annual Meeting 2020, Los Angeles, CA, USA, 22–24 June 2021.
- Park, K.; Jänne, P.A.; Kim, D.W.; Han, J.Y.; Wu, M.F.; Lee, J.S.; Kang, J.H.; Lee, D.H.; Cho, B.C.; Yu, C.J. Olmutinib in T790M-positive non–small cell lung cancer after failure of first-line epidermal growth factor receptor-tyrosine kinase inhibitor therapy: A global, phase 2 study. Cancer 2021, 127, 1407–1416.
- Shi, Y.; Hu, X.; Zhang, S.; Lv, D.; Wu, L.; Yu, Q.; Zhang, Y.; Liu, L.; Wang, X.; Cheng, Y. Efficacy, safety, and genetic analysis of furmonertinib (AST2818) in patients with EGFR T790M mutated non-small-cell lung cancer: A phase 2b, multicentre, single-arm, open-label study. Lancet Respir. Med. 2021, 9, 829–839.
