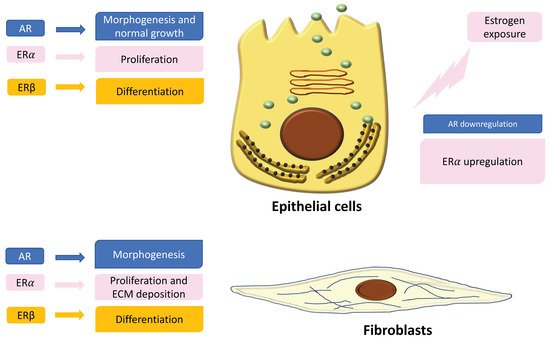
In the normal prostate, AR is the dominant steroid receptor. All the components of the prostate gland—stromal cells, epithelium, and smooth muscle cells—express androgen receptors [
42] (
Figure 1). Interaction between AR and its ligand can strongly guide morphogenesis. On the contrary, it has been shown that estrogens act through multiple ERs, including ERα, ERβ, and GPER, which are expressed in different cell types inside the prostate [
43]. During development, 17β-estradiol (E2) plays a physiologic role in the modulation of branching morphogenesis through the activation of ERα and in the differentiation of prostate epithelium through ERβ [
43,
53,
54,
55,
56]. In particular, lower levels of ERα than AR are localized in stromal cells that surround the proximal ducts during early-life prostate morphogenesis [
43]. ERα significantly declines with puberty as androgen levels rise, suggesting a specific role during development [
43]. Specifically, it has been demonstrated that mouse ERα expressed by different cell types has different actions: fibroblast ERα modulates branching morphogenesis; smooth muscle ERα regulates stromal cell proliferation and deposition of extracellular matrix [
43,
55,
56]. In humans, ERα is expressed by stromal cells during fetal development [
43,
57,
58], and it has been shown that when it is expressed in the periurethral prostatic epithelium during the last gestational period, it is associated with squamous metaplasia [
43,
58] (
Figure 1). Moreover, recently it has been demonstrated that ERα also plays a role in prostatic epithelial stem cells and has involvement in self-renewal and progenitor cell proliferation after estrogen induction [
43,
59,
60,
61]. Different from ERα, rodent ERβ is almost exclusively localized in prostate epithelial cells, and it is involved in differentiation processes of the luminal epithelium [
43,
62]. On the contrary, in humans, ERβ is widely expressed in epithelial and stromal cells by gestational week seven, and it is activated during gestation and for several months after birth, suggesting that ERβ plays a role in development regulation [
43,
57,
58]. Furthermore, ERβ is also localized in stem cells and seems to be involved in progenitor cell differentiation [
43,
61,
63]. Many studies have focused on the central role of steroid receptors in the onset of different prostate pathologies, since it has been shown that they lead to expression and localization changes, initiate growth and differentiation defects during early development, and maintain these phenotypes throughout life [
43]. Indeed, in rodents it has been demonstrated that after exposure to high levels of estrogens during the neonatal critical window (post-natal day PND1-5), ERα and AR immediately change, directly driving the early estrogenized phenotype. Specifically, AR protein is sharply downregulated in both stromal and epithelial cells and remains low throughout life, leading to a reduced response to androgens [
43] (
Figure 1). On the contrary, ERα is upregulated in periductal stromal cells, which in turn permits a transient induction of the prolactin receptor (PRLR) [
43]. Different from ERα and AR, ERβ changes later in development or adulthood [
43]. Thus, the developing prostate is no longer under AR regulation but is rather driven by several estrogens, through different receptors such as ERα and PRLR. The resulting effect is that programming signals that normally guide development of the prostate are altered, leading to permanent alterations in prostate structure and activity throughout life [
43].