Endophytic bacteria reside in the internal tissues of the plant, establishing a strong symbiotic relationship that promotes plant growth and provides protection in exchange for a niche to carry out its life cycle. The nature of their mutualistic association depends on their location in the plant tissue, either intercellularly or intracellularly. Since they promote plant growth, increase crop yields, and afford disease resistance under harsh environmental conditions, endophytic bacteria are considered plant probiotics.
- Banana
- Cavendish
- Endophytic bacteria
- Probiotics
- Sigatoka
- SynComs
1. Introduction
In 2017, bananas were ranked 12th among the top 20 commodities globally, reaching a record production of 116 million tons. Currently, around 5.2 million hectares in 135 countries are dedicated to banana production [1]. Additionally, banana is the fourth most important crop, often recognized as a staple in food security and cash crop for generating income [2]. The most common and widely exported banana is the Cavendish (AAA) group of dessert bananas (Lacatan, Robusta, Valery, Giant Cavendish, Grand Naine, dwarf Cavendish, Petit Naine, and dwarf Parfitt) that account for about 43% of global banana production [1].
Cavendish bananas are grown in nutrient-limited soils under excessive nitrogen (N) fertilization and frequently water-limited conditions [3][4]. In addition, pathogenic fungi, which are becoming increasingly virulent and resistant to fungicides, threaten banana production in major growing areas [4][5][6][7]. Banana plants have a relatively high nutrient and water demand compared to other crops; therefore, applications of high dosages of potassium (K) and N are required in banana orchards to replenish the nutrients exported from the soil to the plant and fruits [8]. It has been reported that an average of 400 Kg N ha −1 Y −1 is used in the Caribbean and Latin American banana orchards, resulting in severe contamination of water bodies [9].
Endophytic bacteria reside in the internal tissues of the plant, establishing a strong symbiotic relationship that promotes plant growth and provides protection in exchange for a niche to carry out its life cycle [10]. The nature of their mutualistic association depends on their location in the plant tissue, either intercellularly or intracellularly [11][12]. Since they promote plant growth, increase crop yields, and afford disease resistance under harsh environmental conditions, endophytic bacteria are considered plant probiotics [13][14][15][16][17].
2. Plant Microbiomes: The Origin of Plant Probiotic Bacteria
Plants have distinct microenvironments that harbor complex and diverse microbial communities, considered a second genome [18]. Plants have selected these microbial communities over millions of years of co-evolution to form a plant-specific microbiome, resulting in various interactions between plants and microorganisms [19]. Additionally, beneficial and detrimental microbial effects on plants can directly or indirectly affect microorganism-microorganism interactions [20].
Beneficial microbes (i.e., rhizospheric and endophytic) improve the acquisition of soil nutrients and tolerance to abiotic stresses and combat pathogens. These functions promote plant growth and consequently increase the ecological fitness of the natural environmental or agricultural system [21]. Endophytic microbes are a subset of the plant microbiome. This group of diverse and heterogeneous bacteria can easily enter plant roots through different mechanisms [22]. It is important to point out that the nature of plant-endophyte interactions can range from mutualism to pathogenicity. It was previously demonstrated that the type of interaction depends on abiotic and biotic factors, including the genotypes of plants and microbes, environmental conditions, and the dynamic networks of interactions within plant biomes [23].
More recently, microbiologists have been using high-throughput sequencing (HTS) methods to explore the structure of bacterial communities, identifying members that cannot be easily cultured in the laboratory [24][25][26], including phyla such as Planctomycetes, Verrucomicrobia, and Acidobacteria [27][28]. A significant proportion of the bacterial genera reported as endophytic is commonly found in the rhizosphere, suggesting that the endophytic microbiome may be a subpopulation of the rhizospheric bacteria [10].
It has been proposed that endophytic bacteria could be used as “plant probiotics” for microbiome reconstruction, improving crop yields, and reducing or even eliminating the requirement for chemical fertilizers [29][30][31]. Additionally, cultured bacterial endophytes display plant growth-promoting (PGP) traits, including nitrogen fixation, nutrient (e.g., nitrogen, phosphate, zinc, and other nutrient elements) and water uptake, and essential phytohormone production (e.g., indole acetic acid (IAA), cytokinin and abscisic acid) [32][33]. These bacteria indirectly provide plants with resistance or tolerance to biotic and abiotic stresses [34][35][36] by upregulating ACC deaminase activity and modulating ethylene biosynthesis [37].
3. Synthetic Communities as Probiotic Bioinoculants
In recent years, significant steps have been taken towards understanding many facets of the plant microbiome and their interactions. With advances in sequencing technologies and analytical tools, we have learned about plant-microbial and microbial-microbial interactions and how microbes are recruited from the environment and assembled into a defined structure. These interactions are widely dependent on soil type, host genotype, and agricultural management [38]. Indeed, these types of studies have altered our perception of the complexity and dynamics of plant-microbe interactions.
Plant microbiomes have been studied by inferred functions derived from descriptive genetic data (metagenomics) and/or combined with metabolomics and culture-dependent approaches to develop synthetic microbial communities (SynComs) [39][40][41]. SynCom research and development involves employing microbial candidates as new functional probiotics for plants [40][42]. According to an analysis of 30 publications, Marin et al. [43] showed that SynComs could range from 3 to 190 microbial strains, mainly composed of bacteria belonging to the phyla Proteobacteria, Actinobacteria, Firmicutes, and Bacteriodetes.
After assembling the microbial consortium, it is then tested in plants to evaluate whether the functions and structure mimic the observed function and structure of the plant microbiome under natural conditions in the time and space of a multidimensional and complex system [38][43]. This approach reduces the complexity of the microbial community without modifying the original interactions among microbes and the host plant (Reviewed in [42][43]). A significant advantage of SynComs is that they are composed of adapting microbial communities with defined and predictable traits for crop management, producing effects that a single microbe could not generate. Additionally, due to the plasticity of SynComs in the laboratory, it is possible to understand how the plant alters its behaviors and genetic responses by removing one or several members of the consortium [43].
Plant microbiome studies are gradually considering the synergistic and cumulative effects of SynComs on different microorganisms, expanding our knowledge of plant diseases [44][45][46][47][48][49][50]. SynComs have also been designed to elucidate the specific function of plant microorganisms, including nutritional aspects (e.g., nitrogen fixation by diazotrophs) [51] and mineral assimilation (e.g., phosphate and organic nitrogen) [52][53]. Thus, the concept of SynComs for creating microbial consortia under laboratory conditions is a promising ecological strategy for developing more resilient and productive crops.
4. The Banana Endophytic Microbiome or Endophytome: History, Diversity, Functionality, and the Cry for Help Phenomena
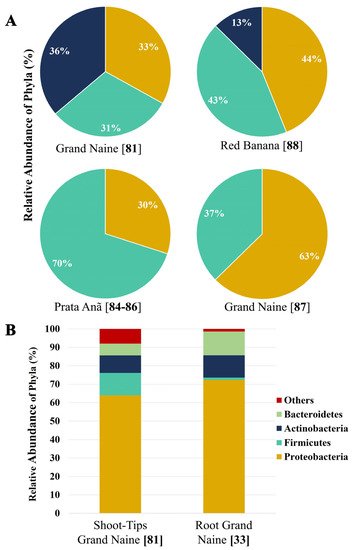
These studies demonstrate that the method of analysis, cultivar, and microhabitat (root, pseudostem, and leaf) are influential factors for endophytic bacteria diversity in banana plants. Figure 1 shows the relative abundance of endophytic bacteria that have been reported in some studies mentioned in this review. The studies were carried out in different tissues of banana plants (Dwarf Cavendish and red banana AAA) and Prata Anã (AAB) varieties grown in commercial plantations exposed to different stress factors (2A). We can also observe the influence of the analysis method (cultivated and non-cultivated) on the distribution of the endophytic microbial community (2B).
Previously, Rudrapa et al. [61] showed that malate efflux induces B. subtilis attraction to Arabidopsis roots during Pseudomonas syringae infection. The attracted B. subtilis triggered systemic resistance and protected plants against P. syringae . The “cry for help” concept was recently supported by a field experiment in which durum wheat naturally infected by Fusarium graminearum was enriched with Stenotrophomonas rhizophila in the rhizospheres and root endospheres to alleviate fungal disease [62]. This protection mechanism has not been described in banana plants. However, evidence shows that the microbial community shifts in banana plants infected by Fusarium , indicating a “cry for help” mechanism.
In another study, previously mentioned in this review [55], a probiotic formulation of endophyte bacterial strains isolated from banana roots under organic management was developed. After a strict selection based on PGP-properties, two strains of Pseudomonas ( P. plecoglossicida and P. taiwanensis ) were found to retard foliar necrosis symptoms in field trials. The control plants presented necrosis symptoms from the first leaf (i.e., the youngest leaf). In contrast, plants treated with endophytic bacteria only showed necrosis symptoms at the fourth leaf (i.e., older). Furthermore, the fruit and the bunch’s average weight was higher in banana plants treated with the endophyte probiotic bacteria.
Our research group has studied black Sigatoka from two scientific approaches to reduce the impact of black Sigatoka in bananas: a) the biochemistry and physiology of P. fijiensis and b) the endophytic bacteria populations and their usefulness as probiotics in commercial plantations of Colima and Jalisco, Mexico [5][14][63][64][65]. First, we proposed E. cloacae , which is widely distributed in banana plants and seeds [14][66][67][68], as a keystone member of the microbial community of these plants. This endophyte serves as a plant-growth promoter, contributes to banana plant nutrition and imparts fungal disease tolerance [56][69][70][71].
This entry is adapted from the peer-reviewed paper 10.3390/microorganisms9091805
References
- Food and Agriculture Organization of the United Nations, Teams on International Investment and Tropical fruits Trade and Market Division. Banana Market Review: Preliminary Results. 2019. Available online: http://www.fao.org/faostat/en/?#data/QC (accessed on 28 June 2021).
- Scott, G.J. A review of root, tuber and banana crops in developing countries: Past, present and future. Int. J. Food Sci. Technol. 2021, 56, 1093–1114.
- Panigrahi, N.; Thompson, A.; Zubelzu, S.; Knox, J. Identifying opportunities to improve management of water stress in banana production. Sci. Hortic. 2020, 276, 109735.
- Meya, A.; Ndakidemi, P.; Mtei, K.; Swennen, R.; Merckx, R. Optimizing soil fertility management strategies to enhance banana production in volcanic soils of the Northern Highlands, Tanzania. Agronomy 2020, 10, 289.
- Aguilar-Barragan, A.; García-Torres, A.; Odriozola-Casas, O.; Macedo-Raygoza, G.; Ogura, T.; Manzo-Sánchez, G.; James, A.; Islas-Flores, I.; Beltran-García, M. Chemical management in fungicide sensivity of Mycosphaerella fijiensis collected from banana fields in México. Braz. J. Microbiol. 2014, 45, 359–364.
- Dita, M.; Barquero, M.; Heck, D.; Mizubuti, E.S.G.; Staver, C.P. Fusarium wilt of banana: Current knowledge on epidemiology and research needs toward sustainable disease management. Front. Plant Sci. 2018, 9, 1468.
- Thangavelu, R.; Saraswathi, M.S.; Uma, S.; Loganathan, M.; Backiyarani, S.; Durai, P.; Raj, E.E.; Marimuthu, N.; Kannan, G.; Swennen, R. Identification of sources resistant to a virulent Fusarium wilt strain (VCG 0124) infecting Cavendish bananas. Sci. Rep. 2021, 11, 3183.
- Raphael, L.; Recous, S.; Ozier-Lafontaine, H.; Sierra, J. Fate of a 15N-labeled urea pulse in heavily fertilized banana crops. Agronomy 2020, 10, 666.
- Aryal, D.R.; Geissen, V.; Ponce-Mendoza, A.; Ramos-Reyes, R.; Becker, M. Water quality under intensive banana production and extensive pastureland in tropical Mexico. J. Soil. Sci. Plant Nutr. 2012, 175, 553–559.
- Vandana, U.K.; Rajkumari, J.; Singha, L.P.; Satish, L.; Alavilli, H.; Sudheer, P.D.V.N.; Chauhan, S.; Ratnala, R.; Satturu, V.; Mazumder, P.B.; et al. The endophytic microbiome as a hotspot of synergistic interactions, with prospects of plant growth promotion. Biology 2021, 10, 101.
- Thomas, P.; Sekhar, A.C. Live cell imaging reveals extensive intracellular cytoplasmic colonization of banana by normally non-cultivable endophytic bacteria. AoB Plants 2014, 6, plu002.
- Thomas, P.; Franco, C.M.M. Intracellular bacteria in Plants: Elucidation of abundant and diverse cytoplasmic bacteria in healthy plant cells using in vitro cell and callus cultures. Microorganisms 2021, 28, 269.
- Beltran-Garcia, M.J.; White, J.F., Jr.; Prado, F.M.; Prieto, K.R.; Yamaguchi, L.F.; Torres, M.S.; Kato, M.J.; Medeiros, M.H.G.; Di Mascio, P. Nitrogen acquisition in Agave tequilana from degradation of endophytic bacteria. Sci. Rep. 2014, 4, 1–7.
- Macedo-Raygoza, G.M.; Valdez, B.S.; Prado, F.M.; Prieto, K.R.; Yamaguchi, L.F.; Kato, M.J.; Canto-Canche, B.B.; Carrillo-Beltran, M.; Di Mascio, P.; White, J.F.; et al. Enterobacter cloacae, an endophyte that establishes a nutrient-transfer symbiosis with banana plants and protects against the black Sigatoka pathogen. Front. Microbiol. 2019, 10, 804.
- Dini-Andreote, F. Endophytes: The second layer of plant defense. Trends Plant Sci. 2020, 25, 319–322.
- Bradshaw, M.J.; Pane, A.M. Field inoculations of nitrogen fixing endophytes on turfgrass. Physiol. Mol. Plant Pathol. 2020, 112, 101557.
- Gupta, S.; White, J.; Kulkarni, M. An outlook on current and future directions in Endophyte research. Editorial Note-Endophyte Special Issue (South African Journal of Botany). S Afr. J. Bot. 2020, 134, 1–2.
- Babalola, O.O.; Fadiji, A.E.; Enagbonma, B.J.; Alori, E.T.; Ayilara, M.S.; Ayangbenro, A.S. The nexus between plant and plant microbiome: Tevelation of the networking strategies. Front. Microbiol. 2020, 11, 548037.
- Ray, P.; Lakshmanan, V.; Labbé, J.L.; Craven, K.D. Microbe to Microbiome: A paradigm shift in the application of microorganisms for sustainable agriculture. Front. Microbiol. 2020, 11, 622926.
- Xiong, W.; Guo, S.; Jousset, A.; Zhao, Q.; Wu, H.; Rong, L.; Kowalchuk, G.; Shen, Q. Bio-fertilizer application induces soil suppressiveness against Fusarium wilt disease by reshaping the soil microbiome. Soil. Biol. Biochem. 2017, 114, 238–247.
- Liu, H.; Carvalhais, L.C.; Crawford, M.; Singh, E.; Dennis, P.G.; Pieterse, C.M.J.; Schenk, P.M. Inner plant values: Diversity, colonization and benefits from endophytic bacteria. Front. Microbiol. 2017, 8, 2552.
- White, J.F.; Kingsley, K.L.; Verma, S.K.; Kowalski, K.P. Rhizophagy cycle: An oxidative process in plants for nutrient extraction from symbiotic microbes. Microorganisms 2018, 6, 95.
- Hardoim, P.R.; van Overbeek, L.S.; Berg, G.; Pirttilä, A.M.; Compant, S.; Campisano, A.; Döring, M.; Sessitsch, A. The hidden world within plants: Ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol. Mol. Biol. Rev. 2015, 79, 293–320.
- Aghdam, S.A.; Brown, A.M.V. Deep learning approaches for natural product discovery from plant endophytic microbiomes. Environ. Microbiome 2021, 16, 1–20.
- Du, X.; Zhai, Y.; Deng, Q.; Tan, H.; Cao, L. Illumina-Based sequencing analysis directed selection for actinobacterial probiotic candidates for banana plants. Probiotics Antimicrob. Proteins 2018, 10, 284–292.
- Ma, Q.; Bücking, H.; Gonzalez Hernandez, J.L.; Subramanian, S. Single-Cell RNA sequencing of plant-associated bacterial communities. Front. Microbiol. 2019, 10, 2452.
- Liu, C.; Dong, Y.; Hou, L.; Deng, N.; Jiao, R. Acidobacteria community responses to nitrogen dose and form in chinese fir plantations in southern China. Curr. Microbiol. 2017, 74, 396–403.
- Fadiji, A.E.; Ayangbenro, A.S.; Babalola, O.O. Metagenomic profiling of the community structure, diversity, and nutrient pathways of bacterial endophytes in maize plant. Antonie Van Leeuwenhoek 2020, 113, 1559–1571.
- Reva, O.N.; Swanevelder, D.Z.H.; Mwita, L.A.; Mwakilili, A.D.; Muzondiwa, D.; Joubert, M.; Chan, W.Y.; Lutz, S.; Ahrens, C.H.; Avdeeva, L.V.; et al. Genetic, epigenetic and phenotypic diversity of four Bacillus velezensis strains used for plant protection or as probiotics. Front. Microbiol. 2019, 10, 2610.
- Mastan, A.; Rane, D.; Dastager, S.G.; Vivek-Babu, C.S. Plant probiotic bacterial endophyte, Alcaligenes faecalis, modulates plant growth and forskolin biosynthesis in Coleus forskohlii. Probiotics Antimicrob. Proteins 2020, 12, 481–493.
- Jayakumar, A.; Padmakumar, P.; Nair, I.; Krishnankutty, R. Drought tolerant bacterial endophytes with potential plant probiotic effects from Ananas comosus. Biologia 2020, 75, 1769–1778.
- Cueva-Yesquén, L.G.; Goulart, M.C.; Attili de Angelis, D.; Nopper Alves, M.; Fantinatti-Garboggini, F. Multiple plant growth-promotion traits in Endophytic Bacteria retrieved in the vegetative stage from passion flower. Front. Plant Sci. 2021, 11, 2282.
- Saha, C.; Mukherjee, G.; Agarwal-Banka, P.; Seal, A. A consortium of non-rhizobial endophytic microbes from Typha angustifolia functions as probiotic in rice and improves nitrogen metabolism. Plant Biol. 2016, 18, 938–946.
- Martínez-Rodríguez, J.; De la Mora-Amutio, M.; Plascencia-Correa, L.A.; Audelo-Regalado, E.; Guardado, F.R.; Hernández-Sánchez, E.; Peña-Ramírez, Y.J.; Escalante, A.; Beltrán-García, M.J.; Ogura, T. Cultivable endophytic bacteria from leaf bases of Agave tequilana and their role as plant growth promoters. Braz. J. Microbiol. 2015, 45, 1333–1339.
- Martinez-Rodriguez, A.; Macedo-Raygoza, G.; Huerta, A.; Reyes-Sepulveda, I.; Lozano-Lopez, J.; García-Ochoa, E.; Fierro-Kong, L.; Medeiros, M.; Di Mascio, P.; White, J.; et al. Agave seed endophytes: Ecology and impacts on root architecture, nutrient acquisition, and cold stress tolerance. Seed Endophytes. Springer 2019, 5, 139–170.
- Kazerooni, E.A.; Maharachchikumbura, S.S.N.; Adhikari, A.; Al-Sadi, A.M.; Kang, S.M.; Kim, L.R.; Lee, I.J. Rhizospheric Bacillus amyloliquefaciens protects Capsicum annuum cv. Geumsugangsan from multiple abiotic stresses via multifarious plant growth-promoting attributes. Front. Plant Sci. 2021, 12, 12.
- Orozco-Mosqueda, M.D.C.; Glick, B.R.; Santoyo, G. ACC deaminase in plant growth-promoting bacteria (PGPB): An efficient mechanism to counter salt stress in crops. Microbiol. Res. 2020, 235, 126439.
- Du, J.; Li, Y.; Yin, Z.; Wang, H.; Zhang, X.; Ding, X. High-throughput customization of plant microbiomes for sustainable agriculture. Front. Plant Sci. 2020, 11, 11.
- Rodriguez, P.; Rothballer, M.; Paul Chowdhury, S.; Nussbaumer, T.; Gutjahr, C.; Falter-Braun, P. Systems biology of plant-microbiome interactions. Mol. Plant 2019, 12, 804–821.
- De Souza, R.S.C.; Armanhi, J.S.L.; Arruda, P. From microbiome to traits: Designing synthetic microbial communities for improved crop resiliency. Front. Plant Sci. 2020, 11, 11.
- Saad, M.; Eida, A.A.; Hirt, H. Tailoring plant-associated microbial inoculants in agricultura—A roadmap for successful application. J. Exp. Bot. 2020, 71, 3878–3901.
- Ke, J.; Wang, B.; Yoshikuni, Y. Microbiome engineering: Synthetic biology of plant-associated microbiomes in sustainable agriculture. Trends Biotechnol. 2021, 39, 244–261.
- Marín, O.; González, B.; Poupin, M.J. From microbial dynamics to functionality in the rhizosphere: A systematic review of the opportunities with synthetic microbial communities. Front. Plant Sci. 2021, 12, 12.
- Ma, K.W.; Niu, Y.; Jia, Y.; Ordon, J.; Copeland, C.; Emonet, A.; Geldner, N.; Guan, R.; Stolze, S.C.; Nakagami, H.; et al. Coordination of microbe-host homeostasis by crosstalk with plant innate immunity. Nat. Plants 2021, 7, 814–825.
- Yin, J.; Yu, Y.; Zhang, Z.; Chen, L.; Ruan, L. Enrichment of potentially beneficial bacteria from the consistent microbial community confers canker resistance on tomato. Microbiol. Res. 2020, 234, 126446.
- Carrión, V.J.; Perez-Jaramillo, J.; Cordovez, V.; Tracanna, V.; de Hollander, M.; Ruiz-Buck, D.; Mendes, L.W.; van Ijcken, W.F.J.; Gomez-Exposito, R.; Elsayed, S.S.; et al. Pathogen-induced activation of disease-suppressive functions in the endophytic root microbiome. Science 2019, 366, 606–612.
- Niu, B.; Paulson, J.; Zheng, X.; Kolter, R. Simplified and representative bacterial community of maize roots. Proc. Natl. Acad. Sci. USA 2017, 114, E2450–E2459.
- Finkel, O.M.; Salas-González, I.; Castrillo, G.; Conway, J.M.; Law, T.F.; Teixeira, P.J.P.L.; Wilson, E.D.; Fitzpatrick, C.R.; Jones, C.D.; Dangl, J.L. A single bacterial genus maintains root growth in a complex microbiome. Nature 2020, 587, 103–108.
- Wu, L.; Yang, B.; Li, M.; Chen, J.; Xiao, Z.; Wu, H.; Tong, Q.; Luo, X.; Lin, W. Modification of rhizosphere bacterial community structure and functional potentials to control Pseudostellaria heterophylla replant disease. Plant Dis. 2020, 104, 25–34.
- Durán, P.; Thiergart, T.; Garrido-Oter, R.; Agler, M.; Kemenm, E.; Schulze-Lefert, P.; Hacquard, S. Microbial interkingdom interactions in roots promote Arabidopsis survival. Cell 2018, 175, 973–983.
- Knoth, J.; Kim, S.H.; Ettl, G.; Doty, S. Biological nitrogen fixation and biomass accumulation within poplar clones as a result of inoculations with diazotrophic endophyte consortia. New Phytol. 2014, 201, 599–609.
- Finkel, O.M.; Salas-González, I.; Castrillo, G.; Spaepen, S.; Law, T.F.; Teixeira, P.J.P.L.; Jones, C.D.; Dangl, J.L. The effects of soil phosphorus content on plant microbiota are driven by the plant phosphate starvation response. PLoS Biol. 2019, 17, e3000534.
- Zhang, J.; Liu, Y.X.; Zhang, N.; Hu, B.; Jin, T.; Xu, H.; Qin, Y.; Yan, P.; Zhang, X.; Guo, X.; et al. NRT1.1B is associated with root microbiota composition and nitrogen use in field-grown rice. Nat. Biotechnol. 2019, 37, 676–684.
- Thomas, P.; Sekhar, A.C. Cultivation Versus Molecular Analysis of Banana (Musa sp.) Shoot-Tip tissue reveals enormous diversity of normally uncultivable endophytic bacteria. Microb. Ecol. 2017, 73, 885–899.
- Marcano, I.E.; Díaz-Alcántara, C.A.; Urbano, B.; González-Andrés, F. Assessment of bacterial populations associated with banana tree roots and development of successful plant probiotics for banana crop. Soil Biol. Biochem. 2016, 99, 1–20.
- Cabanás, G.-L.C.; Fernández-González, A.J.; Cardoni, M.; Valverde-Corredor, A.; López-Cepero, J.; Fernández-López, M.; Mercado-Blanco, J. The Banana root endophytome: Differences between mother plants and suckers and evaluation of selected bacteria to control Fusarium oxysporum f.sp. cubense. J. Fungi 2021, 7, 194.
- Souza, S.; Xavier, A.; Costa, M.; Cardoso, A.; Pereira, M.; Nietsche, S. Endophytic bacterial diversity in banana ‘Prata Anã’ (Musa spp.) roots. Genet. Mol. Biol. 2013, 36, 252–264.
- Andrade, L.F.; de Souza, G.L.; Nietsche, S.; Xavier, A.A.; Costa, M.R.; Cardoso, A.M.; Pereira, M.C.; Pereira, D.F. Analysis of the abilities of endophytic bacteria associated with banana tree roots to promote plant growth. J. Microbiol. 2014, 52, 27–34.
- Pereira, D.; Nietsche, S.; Xavier, A.; Souza, S.; Costa, M.; Duarte, A. Characterization and activity of endophytic bacteria from ‘Prata Anã’ banana crop (Musa sp., AAB). Rev. Ceres 2018, 65, 381–387.
- Karthik, M.; Periyasamy, P.; Ramasamy, K.; Murugaiyan, S. Endophytic bacteria associated with banana cultivars and their inoculation effect on plant growth. J. Hortic. Sci. Biotechnol. 2017, 92, 1–9.
- Rudrappa, T.; Czymmek, K.J.; Paré, P.W.; Bais, H.P. Root-secreted malic acid recruits beneficial soil bacteria. Plant Physiol. 2008, 148, 1547–1556.
- Liu, H.; Li, J.; Carvalhais, L.C.; Percy, C.D.; Prakash Verma, J.; Schenk, P.M.; Singh, B.K. Evidence for the plant recruitment of beneficial microbes to suppress soil-borne pathogens. New Phytol. 2021, 229, 2873–2885.
- Beltrán-García, M.J.; Manzo-Sanchez, G.; Guzmán-González, S.; Arias-Castro, C.; Rodríguez-Mendiola, M.; Avila-Miranda, M.; Ogura, T. Oxidative stress response of Mycosphaerella fijiensis, the causal agent of black leaf streak disease in banana plants, to hydrogen peroxide and paraquat. Can. J. Microbiol. 2009, 55, 887–894.
- Beltran-Garcia, M.J.; Martinez-Rodriguez, A.; Macedo-Raygoza, G.M.; Ortiz-Mendoza, D.; Martinez-Molina, C.; Villalobos-Santana, G. Cepas Bacterianas, Mezcla Probiótica, Nutriente y Método Para la Producción Agrícola. Patent Pending Application PCT/Mx2021/000006 and MX/a/2021/002192, 26 February 2021.
- Lima, A.S.; Prieto, K.R.; Santos, C.S.; Paula-Valerio, H.; Garcia-Ochoa, E.Y.; Huerta-Robles, A.; Beltran-Garcia, M.J.; Di Mascio, P.; Bertotti, M. In-vivo electrochemical monitoring of H2O2 production induced by root-inoculated endophytic bacteria in Agave tequilana leaves. Biosens. Bioelectron. 2018, 99, 108–114.
- Thomas, P.; Swarna, G.K.; Patil, P.; Rawal, R. Ubiquitous presence of normally non-culturable endophytic bacteria in field shoot-tips of banana and their gradual activation to quiescent cultivable form in tissue cultures. Plant Cell Tiss. Organ Cult. 2008, 93, 39–54.
- Costa-Júnior, P.S.P.; Cardoso, F.P.; Martins, A.D.; Teixeira-Buttrós, V.H.; Pasqual, M.; Dias, D.R.; Schwan, R.F.; Dória, J. Endophytic bacteria of garlic roots promote growth of micropropagated meristems. Microbiol Res. 2020, 241, 126585.
- Shastry, R.; Welch, M.; Ravishankar, R.; Ghate, S.; Sandeep, K.; Pd, R. The whole-genome sequence analysis of Enterobacter cloacae strain Ghats1: Insights into endophytic lifestyle-associated genomic adaptations. Arch. Microbiol. 2020, 202, 1571–1579.
- Nakkeeran, S.; Rajamanickam, S.; Saravanan, R.; Vanthana, M.; Soorianathasundaram, K. Bacterial endophytome-mediated resistance in banana for the management of Fusarium wilt. Biotech 2021, 11, 11.
- Köberl, M.; Dita, M.; Martinuz, A.; Staver, C.; Berg, G. Members of Gammaproteobacteria as indicator species of healthy banana plants on Fusarium wilt-infested fields in Central America. Sci. Rep. 2017, 7, 1–9.
- Oljira, A.M.; Hussain, T.; Waghmode, T.R.; Zhao, H.; Sun, H.; Liu, X.; Wang, X.; Liu, B. Trichoderma enhances net photosynthesis, water use efficiency, and growth of wheat (Triticum aestivum L.) under salt stress. Microorganisms 2020, 8, 1565.
