Biofuel, a cost-effective, safe, and environmentally benign fuel produced from renewable sources, has been accepted as a sustainable replacement and a panacea for the damaging effects of the exploration for and consumption of fossil-based fuels.
- biofuel
- biodiesel
- emission
- feedstock
- utilization
- transesterification
- transportation
1. Biofuel as a Renewable Fuel
-
Biofuels are renewable and are carbon- and CO2/GHG-neutral during the progression of the life cycle [17].
2.1. Classification of Biofuels
2.1.1. Classification Based on the Physical State
Solid Biofuels
| Lignocellulosic Biomass | Solid Waste | ||
|---|---|---|---|
| Agricultural Residues | Forest Residues | Energy Crops | |
| Rice straw Rice husk Wheat straw Sorghum straw Corn stover Sugarcane bagasse Sugarcane peel Barley straw Olive pulp Grapeseed |
Firewoods Wood chips Wood branches Sawdust Fruit bunch Willow chips Black locust Pine Spruce Eucalyptus Softwood Hardwood Hybrid poplar |
Switchgrass Miscanthus Energy cane grass Hybrid Pennisetum Triarrhena lutarioriparia Energy cane leaf Energy cane stem Grass leaf Grass stem |
Municipal solid waste Processed paper Plastics Wastewater sludge Food waste Dried animal manure Poultry waste |
Liquid Biofuels
Gaseous Biofuels
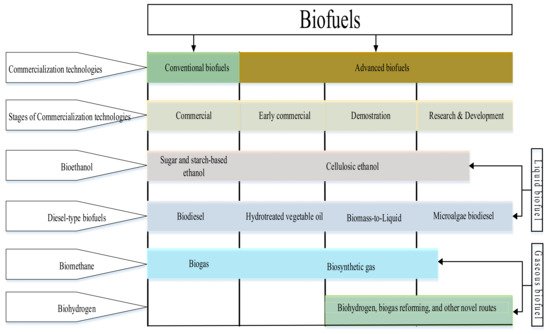
2.1.2. Classification Based on Technology Maturity
2.1.3. Classification Based on the Generation of Feedstock
Feedstocks for biofuel production are divided into three categories in terms of their generation: first-generation feedstock, second-generation feedstock, and third-generation feedstock. The choice of feedstock has a huge influence on the development and utilization of biofuel as a substitute for FB fuels. Feedstocks are chosen based on price, hydrocarbon content, and biodegradability. For example, edible feedstocks and those containing pure sugars are relatively expensive. Simple sugars are preferred as feedstocks because they are easy to decompose with microbes while lignocellulosic biomasses are selected based on their relative affordability.
2.1.4. Classification Based on the Generation of Products
Primary Biofuels
First-Generation Biofuels
Second-Generation Biofuels
Third-Generation Biofuels
Fourth-Generation Biofuels
5. Biofuel as Internal Combustion Engine Fuels
| Fuel | Stored Energy (MJ) |
|---|---|
| Diesel | 36 |
| Gasoline | 33 |
| Biodiesel | 33 |
| Methanol | 16 |
| Ethanol | 21 |
| Liquid H2 (at −253 °C) | 8.5 |
| Compressed H2 (at 250 bar) | 2.5 |
| Property | PBG | PBD | Methanol | Ethanol | DME | Biogas | Hydrogen | Biodiesel | F-T Diesel |
|---|---|---|---|---|---|---|---|---|---|
| Chemical formula | CnH1.87n | CnH1.8n | CH3OH | C2H5OH | CH3OCH3 | CH4 | H2 | C15H31CO2CH3 | C9 to C20 |
| Density (kg/m3) | 720–780 | 820–870 | 800 | 790 | 667 | - | 70 | 850–885 | 774–782 |
| Kinetic viscosity at 40 °C (cSt) | 0.7 | 2.0–3.5 | 0.75 | 1.5 | 0.18 | - | - | 4.43 | 2-4.5 |
| Cetane number | 13–17 | 45–55 | 5 | 8 | 55–60 | - | - | 45-65 | 72 |
| Self-ignition temperature (°C) | 260a | 210 a | 470 | 365 | 320 | 580 | 500 | 220 | 315 |
| Lower heating value (MJ/kg) | 44 | 43 | 19.7 | 28.6 | 28.2 | 24 | 120 | 37 | 43.5 a |
| Lower heating value (liquid) (MJ/L) | 33 | 36 | 16 | 21 | 19 | - | 8.5 | 33 | - |
| Higher heating value (mixture) (kJ/kg) | 3.8 | 3.9 | 3.5 | - | 3.4 | 3.1 | 2.0 | - | - |
| Adiabatic temperature (°C) | 1995 | - | 1950 | 1965 | 2020 | 1954 | 2510 | 2000 | - |
| Boiling temperature (°C) | 25–210 | 180–360 | 65 | 78 | −25 | −162 | −253 | 250–350 | 157.6 |
| Reid vapor pressure at 38 °C (kPa) | 55–100 | <1.5 | 32 | 16 | 800 | - | - | - | - |
| Stoichiometric A/F ratio | 14.5 a | 14 a | 6.4 | 9.0 | 9.0 | 17 | 34.1 | 13 a | 15 |
| Research octane number | 98 | - | 115 | 110 | - | 120 | 106 | - | - |
| Enthalpy of vaporization (kJ/kg) | 350 a | 270 a | 1100 | 900 | 375 | 510 | 455 | - | - |
| Flammability limit (% vol.) | 1.3–8 | 0.6–8 | 7–36 | 4.3–19 | 3.4–19 | - | 4–75 | - | - |
| Flash point (°C) | -40 | 60–80 | 11 | 12 | −41 | - | - | 62 | 500 |
| Oxygen content (wt.%) | - | - | 50 | 35 | 34.8 | - | - | 10.7 | - |
| Carbon content (wt.%) | - | - | - | - | 52.2 | - | - | 76.9 | 86.44 |
| Hydrogen content (wt.%) | - | - | - | - | 13 | - | - | 12.4 | 13.56 |
5.1. Utilization of Biofuels in Spark Ignition Engines
5.2. Utilization of Biofuels in Compression Ignition Engines
| Biofuel Used | Engine Details | Effects | Ref. | |
|---|---|---|---|---|
| Performance | Emissions | |||
| Biodiesel | 1C, 4S, NA, DI, air-cooled |
|
|
[241] |
| Biodiesel | 1C, common-rail DI, r = 16 |
|
|
[242] |
| Biodiesel | 1C, 4S, DI, VCR, water-cooled |
|
|
[243] |
| Biodiesel | 2C, water-cooled, r = 17.5, N = 1500 rpm |
|
|
[244] |
| Biodiesel | 1C, 4S, constant speed, water-cooled |
|
|
[245] |
| DME | 1S, common-rail injection |
|
|
[246] |
| DME | 1S, 4S, DI, water-cooled, r = 18, N = 2200 rpm | NA |
|
[247] |
| DME | 4C, NA, in-line, common rail, r = 18.5 |
|
|
[248] |
| DME | 1S, common rail, r = 16.7 |
|
|
[249] |
| F-T | 1S, 4S, NA, DI, water-cooled, r = 18 |
|
|
[250] |
7. Implications
This entry is adapted from the peer-reviewed paper 10.3390/en14185687