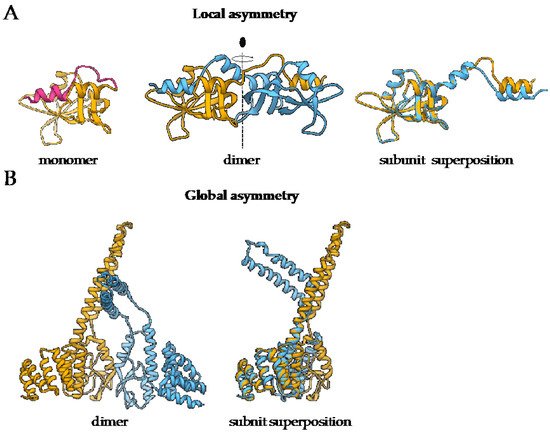
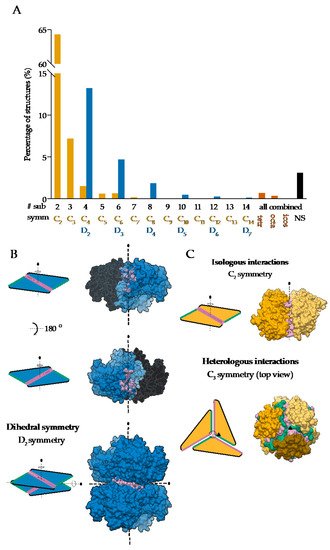
Homo-oligomerization is believed to be nature’s solution to form large proteins by avoiding efficiency problems with the synthesis of long polypeptide chains. In comparison to small proteins, larger ones are more favorable due to their higher stability and smaller solvent-exposed surface percentage. Moreover, building larger protein complexes from smaller subunits has several benefits. Such complexes are less prone to translational errors, as only the defected subunits can be discarded and replaced in contrast to the whole large single polypeptide-chain protein. Next, coding efficiency is higher because less information needs to be stored to build a large protein. Furthermore, assembly of (homo)-oligomeric proteins can be triggered and/or fine-tuned and thus provides an additional layer of regulation, which is crucial in dynamic processes such as actin filament assembly [
3] and microtubule growth [
4]. Other examples are protein activation as a consequence of dimerization, as in the case of caspase-9 activity [
5] and signaling via epidermal growth factor receptor (EGFR) [
6]. Additionally, an opposite effect can be achieved—for example, dimerization inhibits the activity of receptor-like protein tyrosine phosphatase-a [
7]. Even more prominent examples are where the active site is formed at the interface between subunits, as in the case of HIV-1 dimerization protease. [
8]. Besides, oligomerization also enables homotropic allosteric interactions between subunits, for example, in membrane protein αβ TCR [
9] and L-Lactate dehydrogenase [
10]. Such allosteric regulation was found to be the most common in oligomers with dihedral symmetries, especially in metabolic enzymes [
11]. Yet another example are the death domains of several proteins involved in cell death and immune cell signaling where dimerization often leads to protein activation. Here, dimerization is if often mediated via domain swapping where two subunits exchange their parts to form an intertwined dimer [
12]. The same principle can also apply to higher-order homo-oligomers, an example is the barnase domain-swapped trimer [
13], and is also frequently associated with formation of protein aggregates/deposits [
14].
As these advantages are almost intuitive, it is often assumed they should also provide a clear evolutionary benefit. However, Lynch suggested that homo-oligomers could have arisen from stochastic, non-adaptive processes [
15,
16] and that the benefits of homo-oligomerization are not all-pervasive, but rather dependent on the context and the properties of the individual protein [
17]. These and other possible reasons as to why homo-oligomerization is such a frequently encountered property were also extensively discussed elsewhere [
1,
18,
19,
20,
21].