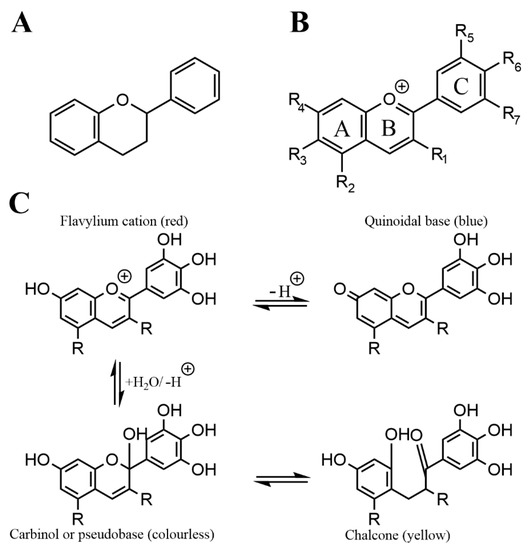
Anthocyanins are a subgroup of flavonoids, which are a prevalent and numerous class of secondary plant metabolites, that all share the C6-C3-C6 flavan or 2-phenyl-benzodihydorpyrane skeleton.
- anthocyanins
- antioxidative
- blood pressure
- hyperlipidemia
1. Overview
|
Food Source |
Anthocyanin Content [mg/100 g fresh weight] |
Reference |
|
Beetroot (Beta vulgaris L.) |
23–77 |
[15] |
|
Bilberry (Vaccinium myrtillus) |
300–698 |
|
|
Black carrots (Daucus carota ssp. sativus var. atrorubens Alef.) |
1.5–190 |
|
|
Blackberry (Rubus fruticosus L) |
83–326 |
|
|
Blackcurrant (Ribes nigrum) |
130–476 |
|
|
Blueberry (Vaccinium angustifolium/ corybosum) |
25–495 |
|
|
Cranberry (Vaccinium macrocarpon) |
46–200 |
|
|
Elderberry (Sambucus nigra L.) |
200–1560 |
|
|
Maqui berry (Aristotelia chilensis) |
137–1250 |
|
|
Pomegranate (Punica granatum) juice |
9–765 mg/L |
|
|
Purple corn (Zea mays indurate) |
68–1642 |
|
|
Red cabbage (Brassica oleracea L. var. capitata L) |
250–322 |
|
|
Red Grape (Vitis vinifera) |
26–750 |
|
|
Redcurrant (Ribes rubrum) |
12–22 |
|
|
Strawberry (Fragaria × ananassa) |
12–55 |
|
|
Tart cherry (Prunus cerasus) |
2–450 |
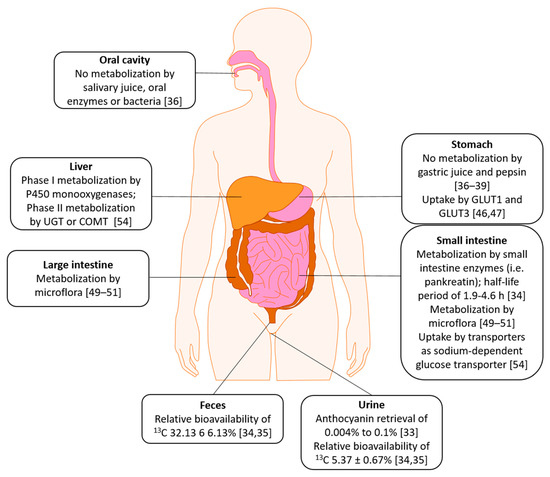
2. Absorption, Distribution, Metabolism, Excretion (ADME)
This entry is adapted from the peer-reviewed paper 10.3390/nu13082831
References
- Castañeda-Ovando, A.; Pacheco-Hernández, M.d.L.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009, 113, 859–871.
- McGhie, T.K.; Walton, M.C. The bioavailability and absorption of anthocyanins: Towards a better understanding. Mol. Nutr. Food Res. 2007, 51, 702–713.
- Kong, J.-M.; Chia, L.-S.; Goh, N.-K.; Chia, T.-F.; Brouillard, R. Analysis and biological activities of anthocyanins. Phytochemistry 2003, 64, 923–933.
- De Pascual-Teresa, S.; Sanchez-Ballesta, M.T. Anthocyanins: From plant to health. J. Phytochem. Rev. 2008, 7, 281–299.
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779.
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A comprehensive review of their chemical properties and health effects on cardiovascular and neurodegenerative diseases. Molecules 2020, 25, 3809.
- Wu, X.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Gebhardt, S.E.; Prior, R.L. Concentrations of anthocyanins in common foods in the United States and estimation of normal consumption. J. Agric. Food Chem. 2006, 54, 4069–4075.
- Horbowicz, M.; Kosson, R.; Grzesiuk, A.; Dębski, H. Anthocyanins of fruits and vegetables—Their occurrence, analysis and role in human nutrition. J. Fruit Ornam. Plant Res. 2008, 68, 5–22.
- Silva, S.; Costa, E.M.; Calhau, C.; Morais, R.M.; Pintado, M.E. Anthocyanin extraction from plant tissues: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 3072–3083.
- Spinardi, A.; Cola, G.; Gardana, C.S.; Mignani, I. Variation of anthocyanin content and profile throughout fruit development and ripening of highbush blueberry cultivars grown at two different altitudes. Front. Plant Sci. 2019, 10, 1045.
- Routray, W.; Orsat, V. Blueberries and their anthocyanins: Factors affecting biosynthesis and properties. Compr. Rev. Food Sci. Food Saf. 2011, 10, 303–320.
- Bar-Ya’akov, I.; Tian, L.; Amir, R.; Holland, D. Primary metabolites, anthocyanins, and hydrolyzable tannins in the pomegranate fruit. Front. Plant Sci. 2019, 10, 620.
- Escribano-Bailón, M.T.; Alcalde-Eon, C.; Muñoz, O.; Rivas-Gonzalo, J.C.; Santos-Buelga, C. Anthocyanins in berries of maqui (Aristotelia chilensis (mol.) stuntz). Phytochem. Anal. PCA 2006, 17, 8–14.
- Fredes, C.; Yousef, G.G.; Robert, P.; Grace, M.H.; Lila, M.A.; Gómez, M.; Gebauer, M.; Montenegro, G. Anthocyanin profiling of wild maqui berries (Aristotelia chilensis [mol.] stuntz) from different geographical regions in chile. J. Sci. Food Agric. 2014, 94, 2639–2648.
- Guiné, R.; Gonçalves, F.; Lerat, C.; Idrissi, T.; Rodrigo, E.; Correia, P.; Gonçalves, J.C. Extraction of phenolic compounds with antioxidant activity from beetroot (Beta vulgaris L.). Curr. Nutr. Food Sci. 2018, 14, 350–357.
- Koponen, J.M.; Happonen, A.M.; Mattila, P.H.; Törrönen, A.R. Contents of anthocyanins and ellagitannins in selected foods consumed in finland. J. Agric. Food Chem. 2007, 55, 1612–1619.
- Lao, F.; Sigurdson, G.T.; Giusti, M.M. Health benefits of purple corn (Zea mays L.) phenolic compounds. Compr. Rev. Food Sci. Food Saf. 2017, 16, 234–246.
- Montilla, E.C.; Arzaba, M.R.; Hillebrand, S.; Winterhalter, P. Anthocyanin composition of black carrot (Daucus carota ssp. Sativus var. Atrorubens alef.) cultivars antonina, beta sweet, deep purple, and purple haze. J. Agric. Food Chem. 2011, 59, 3385–3390.
- Müller, D.; Schantz, M.; Richling, E. High performance liquid chromatography analysis of anthocyanins in bilberries (Vaccinium myrtillus L.), blueberries (Vaccinium corymbosum L.), and corresponding juices. J. Food Sci. 2012, 77, C340–C345.
- Veberic, R.; Jakopic, J.; Stampar, F.; Schmitzer, V. European elderberry (Sambucus nigra L.) rich in sugars, organic acids, anthocyanins and selected polyphenols. Food Chem. 2009, 114, 511–515.
- Yoo, K.S.; Bang, H.; Pike, L.; Patil, B.S.; Lee, E.J. Comparing carotene, anthocyanins, and terpenoid concentrations in selected carrot lines of different colors. Hortic. Environ. Biotechnol. 2020, 61, 385–393.
- Labbe, M.; Ulloa, P.A.; Lopez, F.; Saenz, C.; Pena, A.; Salazar, F.N. Characterization of chemical compositions and bioactive compounds in juices from pomegranates (’wonderful’, ’chaca’ and ’codpa’) at different maturity stages. Chil. J. Agric. Res. 2016, 76, 479–486.
- Fang, J. Some anthocyanins could be efficiently absorbed across the gastrointestinal mucosa: Extensive presystemic metabolism reduces apparent bioavailability. J. Agric. Food Chem. 2014, 62, 3904–3911.
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S.
- Czank, C.; Cassidy, A.; Zhang, Q.; Morrison, D.J.; Preston, T.; Kroon, P.A.; Botting, N.P.; Kay, C.D. Human metabolism and elimination of the anthocyanin, cyanidin-3-glucoside: A 13c-tracer study. Am. J. Clin. Nutr. 2013, 97, 995–1003.
- de Ferrars, R.M.; Czank, C.; Zhang, Q.; Botting, N.P.; Kroon, P.A.; Cassidy, A.; Kay, C.D. The pharmacokinetics of anthocyanins and their metabolites in humans. Br. J. Pharmacol. 2014, 171, 3268–3282.
- Ackermann, M. Studien zum Verhalten von Anthocyanen aus Heidelbeeren im Humanstoffwechsel-Stabilisierung und Bindung Durch Proteine. Ph.D. Thesis, University of Wuerzburg, Wuerzburg, Germany, 3 December 2010.
- Yu, W.; Gao, J.; Hao, R.; Yang, J.; Wei, J. Effects of simulated digestion on black chokeberry (Aronia melanocarpa (michx.) elliot) anthocyanins and intestinal flora. J. Food Sci. Technol. 2021, 58, 1511–1523.
- Bermúdez-Soto, M.J.; Tomás-Barberán, F.A.; García-Conesa, M.T. Stability of polyphenols in chokeberry (Aronia melanocarpa) subjected to in vitro gastric and pancreatic digestion. Food Chem. 2007, 102, 865–874.
- Oliveira, H.; Perez-Gregório, R.; de Freitas, V.; Mateus, N.; Fernandes, I. Comparison of the in vitro gastrointestinal bioavailability of acylated and non-acylated anthocyanins: Purple-fleshed sweet potato vs red wine. Food Chem. 2019, 276, 410–418.
- Kim, I.; Moon, J.K.; Hur, S.J.; Lee, J. Structural changes in mulberry (Morus Microphylla. Buckl) and chokeberry (Aronia melanocarpa) anthocyanins during simulated in vitro human digestion. Food Chem. 2020, 318, 126449.
- Passamonti, S.; Vrhovsek, U.; Mattivi, F. The interaction of anthocyanins with bilitranslocase. Biochem. Biophys. Res. Commun. 2002, 296, 631–636.
- Talavéra, S.; Felgines, C.; Texier, O.; Besson, C.; Gil-Izquierdo, A.; Lamaison, J.L.; Rémésy, C. Anthocyanin metabolism in rats and their distribution to digestive area, kidney, and brain. J. Agric. Food Chem. 2005, 53, 3902–3908.
- Talavéra, S.; Felgines, C.; Texier, O.; Besson, C.; Lamaison, J.L.; Rémésy, C. Anthocyanins are efficiently absorbed from the stomach in anesthetized rats. J. Nutr. 2003, 133, 4178–4182.
- Atnip, A.; Giusti, M.M.; Sigurdson, G.T.; Failla, M.L.; Chitchumroonchokchai, C.; Bomser, J.A. The nci-n87 cell line as a gastric epithelial model to study cellular uptake, trans-epithelial transport, and gastric anti-inflammatory properties of anthocyanins. Nutr. Cancer 2020, 72, 686–695.
- Atnip, A.A.; Sigurdson, G.T.; Bomser, J.; Giusti, M.M. Time, concentration, and ph-dependent transport and uptake of anthocyanins in a human gastric epithelial (nci-n87) cell line. Int. J. Mol. Sci. 2017, 18, 446.
- Oliveira, H.; Fernandes, I.; Brás, N.F.; Faria, A.; De Freitas, V.; Calhau, C.; Mateus, N. Experimental and theoretical data on the mechanism by which red wine anthocyanins are transported through a human mkn-28 gastric cell model. J. Agric. Food Chem. 2015, 63, 7685–7692.
- Han, F.; Oliveira, H.; Brás, N.F.; Fernandes, I.; Cruz, L.; De Freitas, V.; Mateus, N. In vitro gastrointestinal absorption of red wine anthocyanins—Impact of structural complexity and phase ii metabolization. Food Chem. 2020, 317, 126398.
- Mueller, D.; Jung, K.; Winter, M.; Rogoll, D.; Melcher, R.; Kulozik, U.; Schwarz, K.; Richling, E. Encapsulation of anthocyanins from bilberries—Effects on bioavailability and intestinal accessibility in humans. Food Chem. 2018, 248, 217–224.
- Mueller, D.; Jung, K.; Winter, M.; Rogoll, D.; Melcher, R.; Richling, E. Human intervention study to investigate the intestinal accessibility and bioavailability of anthocyanins from bilberries. Food Chem. 2017, 231, 275–286.
- Aura, A.-M.; Martin-Lopez, P.; O’Leary, K.A.; Williamson, G.; Oksman-Caldentey, K.-M.; Poutanen, K.; Santos-Buelga, C. In vitro metabolism of anthocyanins by human gut microflora. Eur. J. Nutr. 2005, 44, 133–142.
- Keppler, K.; Humpf, H.-U. Metabolism of anthocyanins and their phenolic degradation products by the intestinal microflora. Bioorg. Med. Chem. 2005, 13, 5195–5205.
- Faria, A.; Fernandes, I.; Norberto, S.; Mateus, N.; Calhau, C. Interplay between anthocyanins and gut microbiota. J. Agric. Food Chem. 2014, 62, 6898–6902.
- Kamiloglu, S.; Capanoglu, E.; Grootaert, C.; Van Camp, J. Anthocyanin absorption and metabolism by human intestinal caco-2 cells—A review. Int. J. Mol. Sci. 2015, 16, 21555–21574.
- Cassidy, A.; Minihane, A.-M. The role of metabolism (and the microbiome) in defining the clinical efficacy of dietary flavonoids. Am. J. Clin. Nutr. 2016, 105, 10–22.
- Kalt, W.; Liu, Y.; McDonald, J.; Vinqvist-Tymchuk, M.; Fillmore, S.A.E. Anthocyanin metabolites are abundant and persistent in human urine. J. Agric. Food Chem. 2014, 62, 3926–3934.
