Mangrove forests play an important role in mitigating climate change but are threatened by aquaculture expansion (shrimp ponds). The change of land use from natural environments to productive uses, generates a change in the balance and carbon sequestration and storaging. In mangrove forest the carbon stocks are larger than in other tropical forest. Addtionally, soil mangrove forest represent 40-80% of Cardon stocks. These reasons are the evidence of mangrove forest need to be included in REED programs and conservation strategies.
- carbon stocks
- climate change
- mangrove strata
- shrimp ponds
- soil carbon
1. Introduction
2. Mangrove Forest Strata and Shrimp Ponds
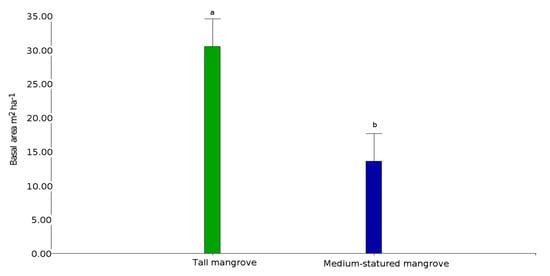
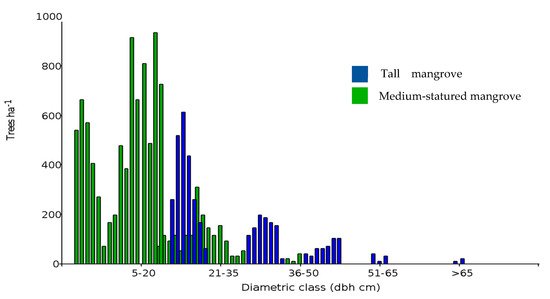
| Mangrove Stratum | Transect | Island | Species | Height | Basal Area | Tree Density |
|---|---|---|---|---|---|---|
| Medium-statured | T11 | San Ignacio | Rhiz | 18.0 ± 2.4 | 10.5 ± 4.3 | 671 ± 281 |
| Medium-statured | T12 | Canoa | Rhiz | 18.2 ± 1.5 | 16.2 ± 6.8 | 942 ± 477 |
| Medium-statured | T13 | San Ignacio | Rhiz | 19.6 ± 3.6 | 16.8 ± 9.0 | 769 ± 271 |
| Medium-statured | T14 | San Ignacio | Rhiz | 16.1 ± 4.1 | 18.5 ± 9.8 | 693 ± 268 |
| Medium-statured | T15 | Chupadores Chico | Rhiz | 8.4 ± 0.8 | 3.2 ± 1.7 | 487 ± 99 |
| Medium-statured | T16 | Chupadores Chico | Rhiz | 13.0 ± 5.1 | 8.8 ± 5.7 | 379 ± 139 |
| Medium-statured | T17 | Chupadores Chico | Rhiz/Lag | 21.1 ± 2.9 | 13.9 ± 4.1 | 531 ± 156 |
| Medium-statured | T18 | Chupadores | Rhiz | 20.8 ± 3.6 | 14.0 ± 2.8 | 498 ± 140 |
| Medium-statured | T19 | San Ignacio | Rhiz | 22.7 ± 1.7 | 27.7 ± 9.9 | 1007 ± 158 |
| Medium-statured | T20 | Canoa | Rhiz | 23.0 ± 4.2 | 23.1 ± 13.0 | 736 ± 168 |
| Medium-statured | T21 | Bellavista | Rhiz | 17.7 ± 1.7 | 20.2 ± 7.9 | 1321 ± 853 |
| Medium-statured | T22 | Chupadores Chico | Rhiz | 12.9 ± 2.2 | 19.2 ± 11.2 | 1039 ± 344 |
| Medium-statured | T4 | Tortuga | Rhiz | 22.9 ± 1.5 | 26.6 ± 8.7 | 1180 ± 255 |
| Medium-statured | T5 | Tortuga | Rhiz/Lag | 19.8 ± 1.4 | 10.1 ± 3.4 | 693 ± 333 |
| Medium-statured | T6 | Tortuga | Rhiz | 18.4 ± 1.6 | 10.0 ± 4.5 | 1061 ± 333 |
| Medium-statured | T7 | Bocana | Rhiz | 14.4 ± 2.7 | 11.1 ± 4.2 | 888 ± 262 |
| Medium-statured | T8 | Bocana | Rhiz/Lag | 16.7 ± 2.9 | 12.0 ± 2.3 | 1288 ± 238 |
| Tall | TM1 | Mondragón | Rhiz | 22.4 ± 6.0 | 32.6 ± 17.9 | 585 ± 179 |
| Tall | TM10 | Mondragón | Rhiz/Lag | 11.8 ± 2.8 | 18.7 ± 9.5 | 985 ± 186 |
| Tall | TM11 | Mondragón | Rhiz/Lag | 14.5 ± 2.1 | 29.2 ± 11.5 | 1245 ± 289 |
| Tall | TM12 | Mondragón | Rhiz | 17.8 ± 2.4 | 34.3 ± 16.8 | 1104 ± 448 |
| Tall | TM3 | Mondragón | Rhiz | 17.3 ± 1.4 | 28.0 ± 14.4 | 660 ± 271 |
| Tall | TM8 | Mondragón | Rhiz | 17.7 ± 3.1 | 31.9 ± 10.0 | 812 ± 365 |
| Tall | TM9 | Mondragón | Rhiz | 23.5 ± 4.7 | 40.9 ± 20.0 | 401 ± 156 |
Note: T and TM = transect code, Rhiz (Rhizophora mangle), Lag (Laguncularia racemosa).
3. Aboveground Carbon
| Seaward Edge (m) | Aboveground Carbon (Mg·ha−1) | |
|---|---|---|
| 25 | 85.8 | a |
| 50 | 86.7 | a |
| 75 | 83.0 | a |
| 100 | 78.3 | a |
| 125 | 59.1 | b |
| 150 | 48.6 | b |
Different letters denote a significant difference (p < 0.05) in mangrove strata.
4. Soil Carbon
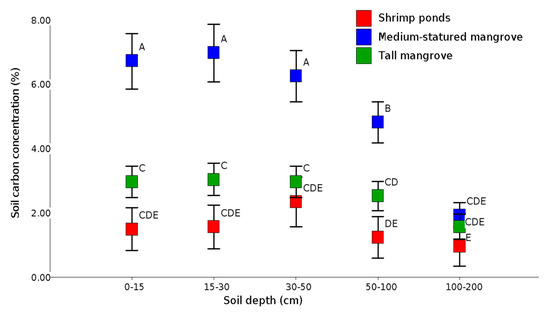
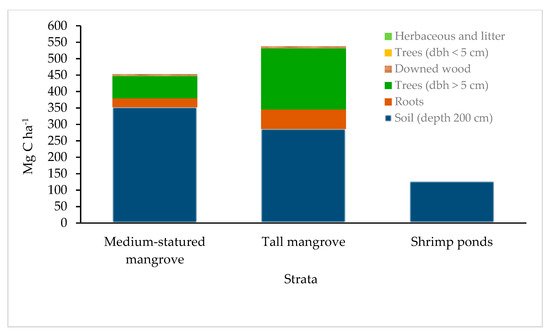
This entry is adapted from the peer-reviewed paper 10.3390/f12070816
References
- Inoue, T. Carbon Sequestration in Mangroves. In Blue Carbon in Shallow Coastal Ecosystems: Carbon Dynamics, Policy, and Implementation; Kuwae, T., Hori, M., Eds.; Springer: Singapore, 2019; pp. 73–99, ISBN 9789811312953.
- Spalding, M.D.; Kainuma, M.; Collins, L. World atlas of mangroves. London, UK: Earthscan. 2010.
- Hamilton, S.E.; Casey, D. Creation of a high spatio-temporal resolution global database of continuous mangrove forest cover for the 21st century (CGMFC-21). Glob. Ecol. Biogeogr. 2016, 25, 729–738.
- Cucalón E. Sistemas Biofísicos y Pesca En El Golfo de Guayaquil: Componente de Oceanografía y Sstemas Físicos. In-forme de Consultoría. Ecuador. 1996, 103 p.
- Adame, M.F.; Kauffman, J.B.; Medina, I.; Gamboa, J.N.; Torres, O.; Caamal, J.P.; Reza, M.; Herrera-Silveira, J.A. Carbon Stocks of Tropical Coastal Wetlands within the Karstic Landscape of the Mexican Caribbean. PLoS ONE 2013, 8, e56569.
- Sanders, C.J.; Smoak, J.M.; Naidu, A.S.; Sanders, L.M.; Patchineelam, S.R. Organic carbon burial in a mangrove forest, margin and intertidal mud flat. Estuar. Coast. Shelf Sci. 2010, 90, 168–172.
- Alongi, D.M. Carbon Cycling and Storage in Mangrove Forests. Annu. Rev. Mar. Sci. 2014, 6, 195–219.
- Alongi, D.M. Carbon Cycling in the World’s Mangrove Ecosystems Revisited: Significance of Non-Steady State Diagene-sis and Subsurface Linkages between the Forest Floor and the Coastal Ocean. Forests 2020, 11, 977.
- Alongi, D.M. Mangrove Forests. In Blue Carbon: Coastal Sequestration for Climate Change Mitigation; Alongi, D.M., Ed.; Springer Briefs in Climate Studies; Springer International Publishing: Cham, Switzerland, 2018; pp. 23–36 ISBN 978-3-319-91698-9.
- Donato, D.C.; Kauffman, J.B.; Murdiyarso, D.; Kurnianto, S.; Stidham, M.; Kanninen, M. Mangroves among the most carbon-rich forests in the tropics. Nat. Geosci. 2011, 4, 293–297.
- Nellemann, C.; Corcoran, E. Blue Carbon: The Role of Healthy Oceans in Binding Carbon : A Rapid Response Assess-ment; UNEP/Earthprint: Norway. 2009; ISBN 978-82-7701-060-1.
- Laumonier, Y.; Edin, A.; Kanninen, M.; Munandar, A.W. Landscape-scale variation in the structure and biomass of the hill dipterocarp forest of Sumatra: Implications for carbon stock assessments. For. Ecol. Manag. 2010, 259, 505–513.
- Atwood, T.B.; Connolly, R.M.; Almahasheer, H.; Carnell, P.E.; Duarte, C.M.; Ewers Lewis, C.J.; Irigoien, X.; Kelleway, J.J.; Lavery, P.S.; Macreadie, P.I.; et al. Global patterns in mangrove soil carbon stocks and losses. Nat. Clim. Chang. 2017, 7, 523–528.
- Valiela, I.; Bowen, J.L.; York, J.K. Mangrove Forests: One of the World’s Threatened Major Tropical Environments: At least 35% of the area of mangrove forests has been lost in the past two decades, losses that exceed those for tropical rain forests and coral reefs, two other well-known threatened environments. BioScience 2001, 51, 807–815.
- Cebrian, J. Variability and control of carbon consumption, export, and accumulation in marine communities. Limnol. Oceanogr. 2002, 47, 11–22.
- Lovelock, C.E.; Ruess, R.W.; Feller, I.C. CO2 Efflux from Cleared Mangrove Peat. PLoS ONE 2011, 6, e21279.
- Giri, C.; Ochieng, E.; Tieszen, L.L.; Zhu, Z.; Singh, A.; Loveland, T.; Masek, J.; Duke, N. Status and distribution of man-grove forests of the world using earth observation satellite data. Glob. Ecol. Biogeogr. 2011, 20, 154–159.
- van der Werf, G.R.; Morton, D.C.; DeFries, R.S.; Olivier, J.G.J.; Kasibhatla, P.S.; Jackson, R.B.; Collatz, G.J.; Randerson, J.T. CO2 emissions from forest loss. Nat. Geosci. 2009, 2, 737–738.
