Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Cell Biology
The evolution of the GC (guanine cytosine) content at the third codon position of the histone genes (H1, H2A, H2B, H3, H4, H2AvD, H3.3A, H3.3B, and H4r) in 12 or more Drosophila species is reviewed. For explaining the evolution of the GC content at the third codon position of the genes, a model assuming selection with a deleterious effect for adenine/thymine and a size effect is presented. The applicability of the model to whole-genome genes is also discussed.
- histone gene
1. Introduction
Histones are basic proteins that package and arrange DNA into nucleosomes [1,2,3,4]. There are two major types of histones: a replication-dependent (canonical) type and a replication-independent (replacement) type [5]. In addition to these, centromeric proteins [6,7,8] and histone-like proteins [9] also exist.
In Drosophila, five replication-dependent (canonical) histones are known [10,11]: H2A, H2B, H3, and H4, which are core histones that organize the nucleosome core by forming an octamer comprising two copies of each protein, and H1, which is a linker protein that binds to each nucleosome core [1,2,3,4]. As for replication-independent (replacement) histones, four kinds are currently known in Drosophila: H2AvD, H3.3A, H3.3B, and H4r [12,13,14,15]. In addition to histone modification [16,17,18,19,20,21,22], the replacement of histones by a different histone type causes chromatin remodeling [23,24,25]. Nucleosome remodeling is involved in many important biological processes, such as cell division, differentiation, gene expression, and replication [26,27,28]. Therefore, histone modification and replacement are mechanisms that can lead to epigenetic changes [21,29,30]. In Drosophila, the histone genes for the canonical type of histones are clustered in a repetitive unit, and in Drosophila melanogaster, the unit repeats about 110 times [10,31]. In contrast, the histone genes for the replacement type of histones are found as single genes or with only a few copies per genome, and they contain a few introns [12,13,14,15]. For the detailed structure of the histone genes in Drosophila, please refer to another review article [32]. The mode of molecular evolution of a multigene family, compared to a single gene, can be studied by analyzing histone genes [33].
The usage of codons in protein-coding genes is not uniform among synonymous codons and is biased in many species [34,35]. The mechanism of codon bias has been discussed for decades, and candidate factors include mutation bias, natural selection, and genetic drift [36,37,38,39,40,41,42,43,44]. Unequal usage of codons occurs when the rate of mutations due to nucleotide substitutions is biased or when selection pressure is exerted differently between synonymous codons. Fitness differences among synonymous codons may be present due to differences in the efficiency or speed of translation [45,46]. However, the selection pressure on codons, if any, would be comparatively weaker than that on amino acid sequences; therefore, the codon usage can be influenced by population size [32,39,47,48,49,50,51,52,53]. Since the largest difference in codon usage is observed in the nucleotide at the third codon position of genes, the guanine–cytosine (GC) content at the third codon position is strongly related to codon usage bias. In Drosophila, the higher the GC content at the third codon position is, the stronger the bias of codons [37,40,54]. Moreover, regarding the relationship with the evolutionary rate, the stronger the bias of codons is, the slower the evolutionary rate [55].
In Drosophila saltans, the low GC content of the Xdh and Adh genes was explained by fluctuating mutation bias [56,57]. However, it may also be explained by changes in selection [32,38,50,51,52,53]. Although many Drosophila species have been analyzed for their histone genes [31,49,58,59,60], no changes in the rate of mutations were observed among the species in our analysis [49]. Here, the evolution of the GC content at the third codon position of histone genes in Drosophila is reviewed, and a model that can best explain the evolution of the GC content at the third codon position in Drosophila is presented.
2. Evolution of the GC Content at the Third Codon Position of the Histone Genes in Drosophila
The GC content at the third codon position of the histone genes in 12 Drosophila species is shown in Figure 1. Parts of histone genes data have been published from our laboratory [31,49,51,52,58,59,60]. The rest is obtained from FlyBase (http://flybase.org, accessed on 2017–2019) [61]. Several characteristic points on the evolution of the GC content at the third codon position of histone genes in Drosophila are summarized below.
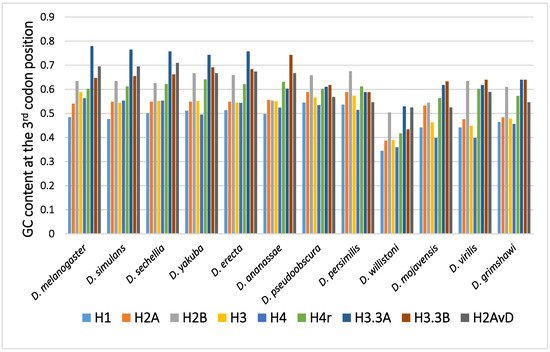
Figure 1. The GC content at the third codon position of the histone genes in Drosophila. The data grouped according to the Drosophila species.
2.1. Disparity in the GC Content at the Third Codon Position among the Genes
In many Drosophila species, the codon usage of the genes was uneven and varied from gene to gene [36,40,50]. Therefore, the GC content at the third codon position differed between the genes. Although the reason remains unclear, codon bias was found to be related to the level of gene expression, which also varied from gene to gene. The positive relationship found between codon bias and the level of gene expression most likely resulted from the difference in translation efficiency [45,46]. Among the canonical histone genes, H2B showed the highest GC content at the third codon position, while H1 showed the lowest GC content at the third codon position [62]. H1, a linker protein, is expressed at approximately half of the level of the other four canonical histones. This is likely the reason why the GC content at the third codon position of H1 is not as high as those of the core histone genes.
2.2. Disparity in the GC Content at the Third Codon Position between the Genes of the Canonical and Replacement Types of Histones
A comparison of the average GC content at the third codon position of genes in 12 common species revealed a higher GC content at the third codon position in the genes of the replacement type of histones than in those of the canonical type of histones [62,63,64,65]. Analysis of codon bias in the histone genes demonstrated that the difference was caused not by an obvious codon bias in a specific amino acid but by a general tendency that was observed for many codons [62]. Differences in functional differentiation or translation efficiency may be the cause of the differences in GC content at the third codon position between the histone types.
2.3. Disparity in GC Content at the Third Codon Position of the Genes among the Different Species
Although variability in the GC content among the genes within a species has been previously noted [36,40,50,51], variability has also been observed between different species [40,51,62]. For example, among 12 Drosophila species, the GC content at the third codon position of many genes in Drosophila willistoni was relatively lower than in the other 11 species [39,62]. Furthermore, when the GC content at the third codon position of corresponding genes was compared between the Drosophila species, nearly parallel differences, similar patterns of ups and downs, were observed for most comparisons (Figure 2). A lower GC content at the third codon position was also observed in the genes of Drosophila species other than these 12 species, such as in Drosophila hydei and Drosophila americana (Figure 1).
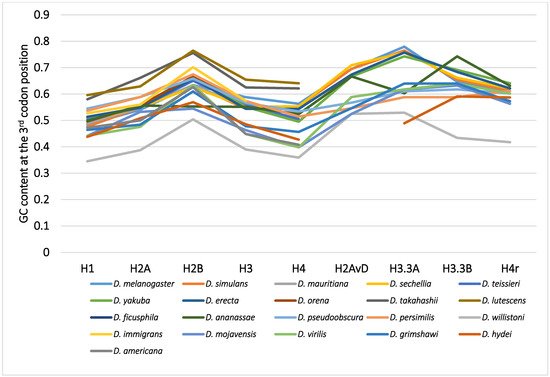
Figure 2. The GC content at the third codon position of the nine histone genes in Drosophila. The points from the same species were connected by lines to show the trend for each species.
2.4. Mode of the Evolution of GC Content at the Third Codon Position According to Phylogeny
The differences in GC content at the third codon position according to the Drosophila phylogeny were unexpected and lacked consistency with evolution [33,62]. The GC contents at the third codon position of closely related species showed similar values, but those in distantly related species did not always show larger differences. Unlike the case for nucleotide and amino acid substitutions, the relationship between differences in GC content at the third codon position and the evolutionary distances between species is not co-linear. The differences in GC content at the third codon position are independent of phylogenetic distance.
This entry is adapted from the peer-reviewed paper 10.3390/genes12050721
This entry is offline, you can click here to edit this entry!
