Gene-editing technology, based on the clustered regularly interspaced short palindromic repeats (CRISPR) platform, has emerged as a revolutionary tool for targeted gene mutation, and has received attention as a game changer in the global biotechnology market.
- upstream open reading frames (uORFs)
- stress-resistant crops
- gene editing
- CRISPR
- enhancing the gene expression
1. Introduction
Crop productivity is increasingly threatened by climate change, and this issue is exacerbated by the growing global population. For example, climate change is causing environmental stress, as exemplified by elevated temperatures, drought, and flooding. On the other hand, global population growth and urbanization has resulted in the loss of agriculturally viable land. According to a study of the correlation between crop yields and global warming, an increase of 1 degree Celsius in global temperatures could result in a reduction of the global production of wheat by 6.0%, rice (Oryza sativa) by 3.2 %, corn (Zea mays) by 7.4 %, and soybeans (Glycine max) by 3.1% [1]. Additionally, it is expected that the global population will reach almost 9 billion by 2050, and the expansion of urbanization will result in an 80% loss of agricultural land by 2030 in Asia and Africa. Consequently, an increase of more than 70% of current crop production will be required to maintain food security [2][3][4].
Genetically modified (GM) crops provide a strategy to increase stress resistance and crop productivity through genetic improvement in relatively short time frames compared to traditional breeding approaches [5]. Furthermore, their generation can enhance the understanding of molecular mechanisms that affect plant development and defense, and this information has provided opportunities to improve crop yield [6][7][8]. However, regulatory hurdles for GM crop commercialization, including safety evaluation, have necessitated the development of alternative strategies involving gene-editing (GE), such as the use of zinc-finger nucleases (ZFNs), TAL effector nucleases (TALENs), and clustered regularly interspaced short palindromic repeats (CRISPR) tools [9]. As an example, Calyxt has developed a TALEN-based genome-edited soybean with modified oil composition and commercialized the derived product, high oleic soybean oil, on the US market [10]. In contrast to the previous GM techniques, which randomly introduce changes, GE allows for precise modification and is identical to those derived from conventional breeding [11][12]. Thus, it is anticipated that many GE crops could be a more acceptable product than GM crops [13].
Messenger RNA (mRNA) is a transcript of the gene, and generally composed of an untranslated region (UTR and intron) and a translated coding region (exon). Upstream open reading frames (uORFs) are a cis-element in the 5’ untranslated regions (UTRs) of mRNAs that induce ribosome stalling and dissociation during the translation of mRNAs [14][15]. Most uORFs negatively regulate the expression of protein encoding main ORF (mORF) of mRNA (Figure 1A). During the translation process, the uORF in the 5’UTR is translated to short peptides, and most uORFs have a start codon (AUG), although some exceptions have been identified [14][16]. It has been predicted by sequence analysis that 49% and 44% of the total transcripts of humans and mice, respectively, have uORFs, as have 20%~40% of the total transcripts of plants such as rice, Arabidopsis thaliana, and corn [17][18]. Most stress-responsive transcripts harboring uORF are induced by specific environmental conditions, and it is known that mutations of uORF sequences reduce their capacity for negative regulation [19][20].
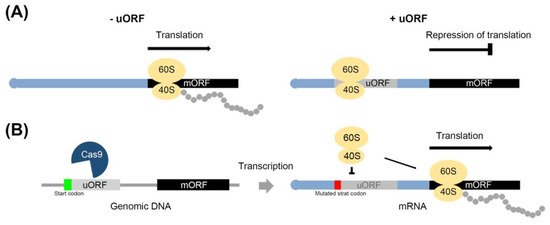
Figure 1. Overview of CRISPR-Cas9-mediated regulation of the repression of translation, as discussed in this review. (A) The mRNA (black rectangle) with uORF (gray rectangle) induces ribosome (yellow ovals) stalling in the uORF, which represses translation of the main ORF (mORF). Polypeptide: gray circle. (B) Mutation of the start codon region (green or red rectangle) in uORF using CRISPR-Cas9 inhibits ribosome stalling, leading to induced translation of mORF.
2. Upstream Open Reading Frames (uORF)
2.1. The Process of Translation of mRNA Mediated by uORF
During mRNA translation, uORF translation causes ribosome stalling, leading to repressed translation of the mORF. This process can be divided into three stages: initiation, elongation, and termination [15][21][22][23][24]. First, the start codon in uORF is recognized by scanning the 40S ribosomal subunit and eukaryotic initiation factors (eIFs), then, in the second stage, the 60S and 80S ribosomal complexes bind to the 40S, allowing for the elongation of the polypeptide chain. Finally, the third stage involves the termination of translation in the uORF, which results in repressed translation of the mORF (Figure 2A). The detailed process can be described in the following steps: (1) ribosome stalling, physical interaction between the peptide encoded by the uORF and ribosomes; (2) the translational machinery dissociates from the mRNA after uORF translation and the mORF is not translated; (3) trigger mRNA decay, nonsense-mediated mRNA decay (NMD) is induced due to the presence of the stop codons in uORF and the recognition as premature transcript; and (4) decreasing mORF translation, after uORF translation elongation, the 40S and 60S dissociate from the uORF, and the 40S unit remains associated with the mRNA. Translation of the mORF is then re-initiated.
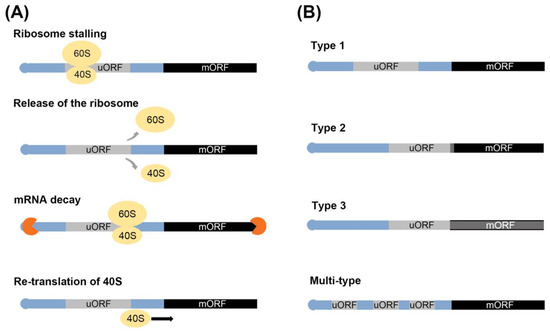
Figure 2. Regulation of uORF-mediated translation and types of uORF. (A) The various mechanism of translational repression. (B) Types of uORFs. uORFs are classified multiple type and type 1–3. Blue, gray and black rectangles indicate regions of the mRNA, yellow ovals indicate the ribosome complex, and orange circles indicate the exosome.
2.2. The Classification of uORF
The uORFs are classified by the position of the uORF stop codon [25]. Type 1: the location of the stop codon of the uORF is independent of the mORF. Type 2: the stop codon of the uORF overlaps with the mORF. Type 3: the stop codon of the uORF is the same as that of the stop codon of the mORF (Figure 2B). In rice, it was expected that uORF containing genes are 20.65% of the total genes. Type 1 and Type 2 account for 87.63% and 12.17% of the total uORFs, respectively, and there are only 41 examples of Type 3 uORFs [18][25].
2.3. Stress-Responsive uORF-Mediated Transcripts in Rice
Drought and the salinization of soils are accelerated by climate change and lead to a significant reduction in crop yield [26][27]. These environmental phenomena cause cellular dehydration by reducing the water content of the cytoplasm, leading to substantial physiological and cytological changes [28][29][30]. To survive under such stressful conditions, plants have developed a range of mechanisms to reduce the effects of drought stress, many of which are associated with extensive changes in gene expression [31][32]. Notably, it was reported that most stress-responsive transcripts harbor uORFs in plants [21][25]. Rice is an excellent model to study the significance of uORFs in stress-related gene expression since its genome has been extensively characterized, it is a major crop, and it is sensitive to drought and salt stress [33][34]. As part of a mechanism associated with salt stress, members of the chloride channel protein (CLC) family, which is found in prokaryotes and eukaryotes, play an important role in ion homeostasis [35][36]. A characterized example in rice is the OsCLC1 protein, which is located at the tonoplast in the cell [37], and overexpression of OsCLC1 in rice was found to enhance drought stress tolerance through modulating jasmonic acid (JA) signaling [38]. We predicted the uORF in OsCLC1 transcripts using uORFFlight (http://uorflight.whu.edu.cn accessed on 20 February 2021) and identified a type 1 uORF in the 5’UTR. The uORF is comprised of 33 nucleotides, including the start codon (ATG) and stop codon (TGG). We also analyzed the uORFs in the 5’UTRs of the salt stress-responsive rice genes through the identification of 107 salt stress-responsive genes reported in the literature, and predicted 393 uORFs in the 46 transcripts related to the transporter, transcription factor, and kinase functional categories. Of these, type 1 uORFs accounted for 91%, while only 8.7% were of type 2, and type 3 uORFs were not represented.
2.4. Avoidance of uORF-Mediated Repression of Gene Expression during Stress Responses
Under stressful conditions, plants activate their defense systems and limit their growth, which is associated with resource restrictions [39][40]. The interaction between defense and growth mechanisms in plants involves in a well-documented tradeoff system. Antagonistic interactions between JA- and gibberellic acid (GA)-regulated processes are one of the cases [41][42][43]. uORF-mediated translational control represents another instance of a tradeoff system. For example, the expression of uORF-associated transcripts is down-regulated by uORF translation under normal conditions to facilitate plant growth. To upregulate their expression under stress conditions, strategies are employed to avoid uORF-mediated translation. These include leaky scanning of the uORF and the reinitiation of the translation process, and the alternative splicing and selection of the transcription start site during the transcription process [14][43][44][45][46][47]. (1) Leaky scanning of uORF: 40S ribosome subunits initiate the translation on the mRNA 5’ cap and recognize the uORF before the mORF (Figure 1A). Unlike regular scanning, 40S ribosome subunits do not scan the uORF start codon, and recognize the mORF start codon through leaky scanning. (2) Reinitiation of translation: After translation of the uORF, the 60S ribosome subunits are released, and only 40S ribosome subunits bind to the mORF (Figure 2A). When mORF translation is required, the remaining 40S ribosome subunits in mRNA recognize the mORF start codon and associate with the 60S subunits again. (3) Alternative slicing: the uORF start codon or uORF sequences in the intron are removed by the splicing of pre-mRNA. The transcript without the uORF is generated from pre-mRNA (Figure 3A). (4) Selection of the transcription start sites: the transcripts of numerous genes are determined by multiple transcription start sites (TSSs). Under normal conditions, transcripts with a uORF are produced from TSSs located upstream of the uORF, whereas under stress conditions, transcripts without a uORF are produced from TSSs located downstream of the uORF (Figure 3B).
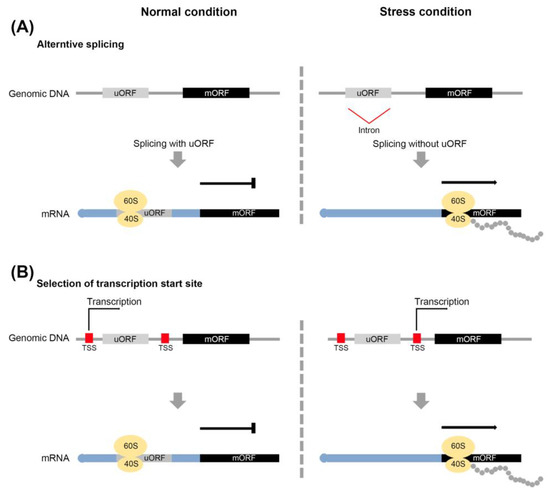
Figure 3. Mechanism of avoiding uORF-mediated repression of gene expression. Alternative splicing (A) and alternative transcription start site (TSS) (B) to exclude a uORF from mRNA.
3. Genetically Modified (GM) Crops
Various strategies have been used to develop biotech crops, including GM crops, which are projected to be important for meeting crop yield targets and food security goals [48][49]. The development of GM crops began in the 1980s and focused on the insertion of useful genes into plant genomes or the suppressed expression of undesirable genes through genetic engineering coupled with innovative plant transformation techniques [50][51][52]. Plant genetic engineering was further accelerated by discovery-based studies of model experimental species, such as Arabidopsis thaliana [53]. The alteration or introduction of agronomically important traits, such as insect resistance or herbicide tolerance, can be achieved through the insertion of a foreign gene into the plant genome. Indeed, the first generation of transgenic crops included corn, soybean, and cotton lines, into which bacterial genes were introduced to confer herbicide resistance or pest resistance [54][55][56]. The development of such crops’ tolerance has led to reductions in the application of pesticides and an expansion of weed management options [57][58].
4. Enhancing Gene Expression in Crops by Editing uORFs Using the CRISPR System as an Alternative to GM Approaches
Over the last few decades, GM crops have been developed based on the functional study of individual genes. However, the average time and cost for developing GM crops is 13 years and $130 million, respectively, and in many countries, regulatory obstacles still block the commercial cultivation of GM crops [59]. Recently, GE technology, which involves the precise modification of the target genome locus, has enabled the development of bio-engineered crops at a lower cost and in a shorter time period [60][61]. However, this approach has inherent limitations in terms of target gene selection compared to GM technology. GM technology was applied for both overexpression and repression of the target genes. However, GE technology has mainly been applied so far for generating the mutants of target genes by mutation and deletion. Here, we highlight the potential for editing uORFs using GE technology to enhance the expression of target genes associated with various crop traits, thereby expanding the scope of target gene selection and allowing for precise control of target gene expression. For example, editing of the uORF of LsGGP2, which encodes an enzyme involved in vitamin C biosynthesis, using the CRISPR-Cas9 system conferred enhanced oxidative stress tolerance and elevated ascorbate levels [62]. In addition, editing the conserved uORF of FvebZIP1.1, which fine-tunes carbon–nitrogen metabolism, resulted in enhanced sugar content in strawberries compared to wild-type [63]
Generally, elevated expression of stress-related genes inhibits plant growth due to the tradeoff between growth and defense. For example, overexpression of AtJMT in rice led to drought tolerance but decreased growth and yield [64]. Many salt stress-responsive genes, including HAK1, NHX1, SOS1, IDS1, NAC6, EIL2, bHLH035, JAZ9, RSS3, ACA6, and ABA2, have been identified and functionally characterized by transgenic research. The editing of uORFs in crops using GE technology to target such genes with a verified function associated with stress resistance would be expected to save time and incur less financial cost than traditional breeding or GM approaches, and this strategy is of growing interest [65].
We summarize the process of gene editing of uORFs with the CRISPR system for the development of GE crops (Figure 4): (1) screening mutants or genes for improving the crop traits of interest, such as salt stress-resistance; (2) investigating the gene function and traits, then isolating the candidate genes; (3) analysis and prediction of uORFs in the 5’UTR of the selected gene; (4) investigating the predicted uORFs using a dual-luciferase assay in protoplasts; (5) constructing a CRISPR vector for targeting uORFs and generating the uORF mutant; (6) assessing altered expression of the target gene by uORF mutation and selecting the homozygous mutants without transgene by genotyping; and (7) characterizing the GE crop traits under field conditions.
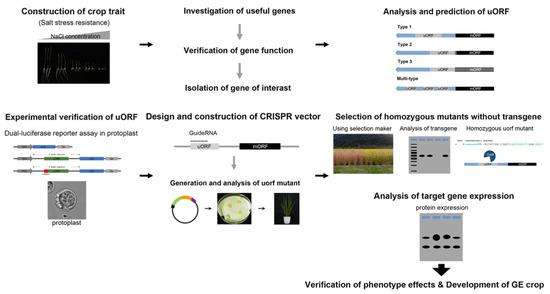
Figure 4. Overview of the process of gene editing of uORFs with the CRISPR system for the development of GE crops.
This entry is adapted from the peer-reviewed paper 10.3390/ijms22073743
References
- Zhao, C.; Liu, B.; Piao, S.; Wang, X.; Lobell, D.B.; Huang, Y.; Huang, M.; Yao, Y.; Bassu, S.; Ciais, P.; et al. Temperature increase reduces global yields of major crops in four independent estimates. Proc. Natl. Acad. Sci. USA 2017, 114, 9326–9331.
- d’Amour, C.B.; Reitsma, F.; Baiocchi, G.; Barthel, S.; Güneralp, B.; Erb, K.-H.; Haberl, H.; Creutzig, F.; Seto, K.C. Future urban land expansion and implications for global croplands. Proc. Natl. Acad. Sci. USA 2017, 114, 8939–8944.
- Ray, D.K.; Mueller, N.D.; West, P.C.; Foley, J.A. Yield trends are insufficient to double global crop production by 2050. PLoS ONE 2013, 8, e66428.
- Röös, E.; Bajželj, B.; Smith, P.; Patel, M.; Little, D.; Garnett, T. Greedy or needy? Land use and climate impacts of food in 2050 under different livestock futures. Glob. Environ. Chang. 2017, 47, 1–12.
- Kamthan, A.; Chaudhuri, A.; Kamthan, M.; Datta, A. Genetically modified (GM) crops: Milestones and new advances in crop improvement. Theor. Appl. Genet. 2016, 129, 1639–1655.
- Sakamoto, T. Phytohormones and rice crop yield: Strategies and opportunities for genetic improvement. Transgenic Res. 2006, 15, 399–404.
- McSteen, P.; Zhao, Y. Plant hormones and signaling: Common themes and new developments. Dev. Cell 2008, 14, 467–473.
- Spartz, A.K.; Gray, W.M. Plant hormone receptors: New perceptions. Genes Dev. 2008, 22, 2139–2148.
- Gaj, T.; Gersbach, C.A.; Barbas, C.F., III. ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 2013, 31, 397–405.
- Calyxt Inc. First Commercial Sale of Calyxt High Oleic Soybean Oil on the U.S. Market; Calyxt Inc.: St. Paul, MN, USA, 2019; Available online: (accessed on 26 February 2019).
- Sikora, P.; Chawade, A.; Larsson, M.; Olsson, J.; Olsson, O. Mutagenesis as a tool in plant genetics, functional genomics, and breeding. Int. J. Plant Genom. 2011, 2011, 314829.
- Grohmann, L.; Keilwagen, J.; Duensing, N.; Dagand, E.; Hartung, F.; Wilhelm, R.; Bendiek, J.; Sprink, T. Detection and identification of genome editing in plants: Challenges and opportunities. Front. Plant Sci. 2019, 10, 236.
- Menz, J.; Modrzejewski, D.; Hartung, F.; Wilhelm, R.; Sprink, T. Genome edited crops touch the market: A view on the global development and regulatory environment. Front. Plant Sci. 2020, 11, 586027.
- Kurihara, Y. uORF Shuffling Fine-Tunes Gene Expression at a Deep Level of the Process. Plants 2020, 9, 608.
- Morris, D.R.; Geballe, A.P. Upstream open reading frames as regulators of mRNA translation. Mol. Cell. Biol. 2000, 20, 8635–8642.
- Bazin, J.; Baerenfaller, K.; Gosai, S.J.; Gregory, B.D.; Crespi, M.; Bailey-Serres, J. Global analysis of ribosome-associated noncoding RNAs unveils new modes of translational regulation. Proc. Natl. Acad. Sci. USA 2017, 114, E10018–E10027.
- Calvo, S.E.; Pagliarini, D.J.; Mootha, V.K. Upstream open reading frames cause widespread reduction of protein expression and are polymorphic among humans. Proc. Natl. Acad. Sci. USA 2009, 106, 7507–7512.
- Von Arnim, A.G.; Jia, Q.; Vaughn, J.N. Regulation of plant translation by upstream open reading frames. Plant Sci. 2014, 214, 1–12.
- Ebina, I.; Takemoto-Tsutsumi, M.; Watanabe, S.; Koyama, H.; Endo, Y.; Kimata, K.; Igarashi, T.; Murakami, K.; Kudo, R.; Ohsumi, A. Identification of novel Arabidopsis thaliana upstream open reading frames that control expression of the main coding sequences in a peptide sequence-dependent manner. Nucleic Acids Res. 2015, 43, 1562–1576.
- Hayashi, N.; Sasaki, S.; Takahashi, H.; Yamashita, Y.; Naito, S.; Onouchi, H. Identification of Arabidopsis thaliana upstream open reading frames encoding peptide sequences that cause ribosomal arrest. Nucleic Acids Res. 2017, 45, 8844–8858.
- Barbosa, C.; Peixeiro, I.; Romão, L. Gene expression regulation by upstream open reading frames and human disease. PLoS Genet. 2013, 9, e1003529.
- Wittmann, J.; Hol, E.M.; Jäck, H.-M. hUPF2 silencing identifies physiologic substrates of mammalian nonsense-mediated mRNA decay. Mol. Cell. Biol. 2006, 26, 1272–1287.
- Yepiskoposyan, H.; Aeschimann, F.; Nilsson, D.; Okoniewski, M.; Mühlemann, O. Autoregulation of the nonsense-mediated mRNA decay pathway in human cells. RNA 2011, 17, 2108–2118.
- Spriggs, K.A.; Bushell, M.; Willis, A.E. Translational regulation of gene expression during conditions of cell stress. Mol. Cell 2010, 40, 228–237.
- Niu, R.; Zhou, Y.; Zhang, Y.; Mou, R.; Tang, Z.; Wang, Z.; Zhou, G.; Guo, S.; Yuan, M.; Xu, G. uORFlight: A vehicle toward uORF-mediated translational regulation mechanisms in eukaryotes. Database (Oxford) 2020, 2020.
- Mittler, R. Abiotic stress, the field environment and stress combination. Trends Plant Sci. 2006, 11, 15–19.
- Peet, M.; Kramer, P. Effects of decreasing source/sink ratio in soybeans on photosynthesis, photorespiration, transpiration and yield. Plant Cell Environ. 1980, 3, 201–206.
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250.
- Bartels, D.; Sunkar, R. Drought and salt tolerance in plants. Crit. Rev. Plant Sci. 2005, 24, 23–58.
- Verslues, P.E.; Agarwal, M.; Katiyar-Agarwal, S.; Zhu, J.; Zhu, J.K. Methods and concepts in quantifying resistance to drought, salt and freezing, abiotic stresses that affect plant water status. Plant J. 2006, 45, 523–539.
- Wellman, C.H.; Gray, J. The microfossil record of early land plants. Philos. Trans. R. Soc. B Biol. Sci. 2000, 355, 717–732.
- Wellman, C.H.; Osterloff, P.L.; Mohiuddin, U. Fragments of the earliest land plants. Nature 2003, 425, 282.
- Eckardt, N.A. Sequencing the rice genome. Am. Soc. Plant Biol. 2000.
- Fairhurst, T.; Dobermann, A. Rice in the global food supply. World 2002, 5, 454, 349–511, 675.
- Mindell, J.; Maduke, M. ClC chloride channels. Genome Biol. 2001, 2, reviews3003.1.
- Jentsch, T.J. CLC chloride channels and transporters: From genes to protein structure, pathology and physiology. Crit. Rev. Biochem. Mol. Biol. 2008, 43, 3–36.
- Nakamura, A.; Fukuda, A.; Sakai, S.; Tanaka, Y. Molecular cloning, functional expression and subcellular localization of two putative vacuolar voltage-gated chloride channels in rice (Oryza sativa L.). Plant Cell Physiol. 2006, 47, 32–42.
- Um, T.Y.; Lee, S.; Kim, J.-K.; Jang, G.; Do Choi, Y. CHLORIDE CHANNEL 1 promotes drought tolerance in rice, leading to increased grain yield. Plant Biotechnol. Rep. 2018, 12, 283–293.
- Huot, B.; Yao, J.; Montgomery, B.L.; He, S.Y. Growth–defense tradeoffs in plants: A balancing act to optimize fitness. Mol. Plant 2014, 7, 1267–1287.
- Züst, T.; Agrawal, A.A. Trade-offs between plant growth and defense against insect herbivory: An emerging mechanistic synthesis. Annu. Rev. Plant Biol. 2017, 68, 513–534.
- Hou, X.; Ding, L.; Yu, H. Crosstalk between GA and JA signaling mediates plant growth and defense. Plant Cell Rep. 2013, 32, 1067–1074.
- Yang, D.-L.; Yao, J.; Mei, C.-S.; Tong, X.-H.; Zeng, L.-J.; Li, Q.; Xiao, L.-T.; Sun, T.-P.; Li, J.; Deng, X.-W. Plant hormone jasmonate prioritizes defense over growth by interfering with gibberellin signaling cascade. Proc. Natl. Acad. Sci. USA 2012, 109, E1192–E1200.
- Um, T.Y.; Lee, H.Y.; Lee, S.; Chang, S.H.; Chung, P.J.; Oh, K.-B.; Kim, J.-K.; Jang, G.; Choi, Y.D. JASMONATE ZIM-DOMAIN PROTEIN 9 interacts with SLENDER RICE 1 to mediate the antagonistic interaction between jasmonic and gibberellic acid signals in rice. Front. Plant Sci. 2018, 9, 1866.
- van der Horst, S.; Filipovska, T.; Hanson, J.; Smeekens, S. Metabolite control of translation by conserved peptide uORFs: The ribosome as a metabolite multisensor. Plant Physiol. 2020, 182, 110–122.
- Kozak, M. Effects of intercistronic length on the efficiency of reinitiation by eucaryotic ribosomes. Mol. Cell. Biol. 1987, 7, 3438–3445.
- Srivastava, A.K.; Lu, Y.; Zinta, G.; Lang, Z.; Zhu, J.-K. UTR-dependent control of gene expression in plants. Trends Plant Sci. 2018, 23, 248–259.
- Tokizawa, M.; Kusunoki, K.; Koyama, H.; Kurotani, A.; Sakurai, T.; Suzuki, Y.; Sakamoto, T.; Kurata, T.; Yamamoto, Y.Y. Identification of Arabidopsis genic and non-genic promoters by paired-end sequencing of TSS tags. Plant J. 2017, 90, 587–605.
- Ahmad, P.; Ashraf, M.; Younis, M.; Hu, X.; Kumar, A.; Akram, N.A.; Al-Qurainy, F. Role of transgenic plants in agriculture and biopharming. Biotechnol. Adv. 2012, 30, 524–540.
- He, Z.; Xia, X.; Peng, S.; Lumpkin, T.A. Meeting demands for increased cereal production in China. J. Cereal Sci. 2014, 59, 235–244.
- Barton, K.A.; Binns, A.N.; Matzke, A.J.; Chilton, M.-D. Regeneration of intact tobacco plants containing full length copies of genetically engineered T-DNA, and transmission of T-DNA to R1 progeny. Cell 1983, 32, 1033–1043.
- Gatehouse, J.A. Biotechnological prospects for engineering insect-resistant plants. Plant Physiol. 2008, 146, 881–887.
- Herrera-Estrella, L.; Depicker, A.; Van Montagu, M.; Schell, J. Expression of chimaeric genes transferred into plant cells using a Ti-plasmid-derived vector. Nature 1983, 303, 209.
- Meinke, D.W.; Cherry, J.M.; Dean, C.; Rounsley, S.D.; Koornneef, M. Arabidopsis thaliana: A model plant for genome analysis. Science 1998, 282, 662–682.
- Gordon-Kamm, W.J.; Spencer, T.M.; Mangano, M.L.; Adams, T.R.; Daines, R.J.; Start, W.G.; O’Brien, J.V.; Chambers, S.A.; Adams, W.R.; Willetts, N.G. Transformation of maize cells and regeneration of fertile transgenic plants. Plant Cell 1990, 2, 603–618.
- McCabe, D.E.; Swain, W.F.; Martinell, B.J.; Christou, P. Stable transformation of soybean (Glycine max) by particle acceleration. Bio/Technology 1988, 6, 923.
- Umbeck, P.; Johnson, G.; Barton, K.; Swain, W. Genetically transformed cotton (Gossypium hirsutum L.) plants. Bio/Technology 1987, 5, 263.
- Cerdeira, A.L.; Duke, S.O. The current status and environmental impacts of glyphosate-resistant crops. J. Environ. Qual. 2006, 35, 1633–1658.
- Raven, P.H. Does the use of transgenic plants diminish or promote biodiversity? New Biotechnol. 2010, 27, 528–533.
- Le Page, M. The second great food war. New Sci. 2018, 239, 22–23.
- Gaj, T.; Sirk, S.J.; Shui, S.-L.; Liu, J. Genome-editing technologies: Principles and applications. Cold Spring Harb. Perspect. Biol. 2016, 8, a023754.
- Ahmar, S.; Saeed, S.; Khan, M.H.U.; Ullah Khan, S.; Mora-Poblete, F.; Kamran, M.; Faheem, A.; Maqsood, A.; Rauf, M.; Saleem, S. A Revolution toward Gene-Editing Technology and Its Application to Crop Improvement. Int. J. Mol. Sci. 2020, 21, 5665.
- Zhang, H.; Si, X.; Ji, X.; Fan, R.; Liu, J.; Chen, K.; Wang, D.; Gao, C. Genome editing of upstream open reading frames enables translational control in plants. Nat. Biotechnol. 2018, 36, 894–898.
- Xing, S.; Chen, K.; Zhu, H.; Zhang, R.; Zhang, H.; Li, B.; Gao, C. Fine-tuning sugar content in strawberry. BMC Genome Biol. 2020, 21, 230.
- Kim, E.H.; Kim, Y.S.; Park, S.-H.; Koo, Y.J.; Do Choi, Y.; Chung, Y.-Y.; Lee, I.-J.; Kim, J.-K. Methyl jasmonate reduces grain yield by mediating stress signals to alter spikelet development in rice. Plant Physiol. 2009, 149, 1751–1760.
- Si, X.; Zhang, H.; Wang, Y.; Chen, K.; Gao, C. Manipulating gene translation in plants by CRISPR–Cas9-mediated genome editing of upstream open reading frames. Nat. Protoc. 2020, 15, 338–363.
