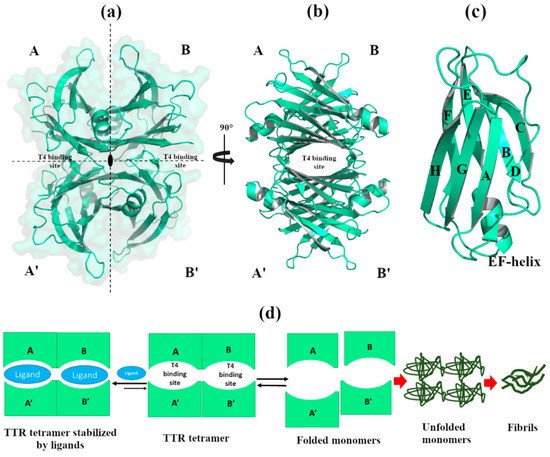
2.2.1. Zn2+
Several studies report that Zn
2+ binds to TTR, both in vitro and in vivo [
28,
37,
38]. High levels of Zn
2+ and Cu
2+ ions induce the formation of TTR amyloid deposits in vitro, while chelating agents favor their disruption. In ex-vivo ocular amyloid deposits, from FAP patients with the V30M mutation, elevated concentrations of Zn
2+ was found [
37]. Starting from this evidence, it was hypothesized that Zn
2+, binding with TTR, might induce structural changes triggering its amyloidogenesis process.
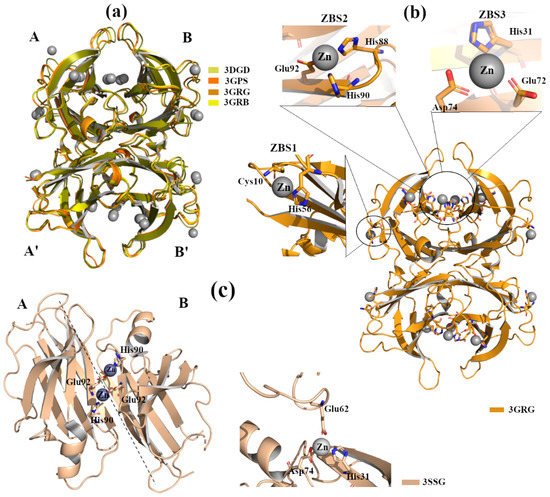
X-ray crystal structure analyses of four engineered monomer TTRs (M-TTR, F87M/L110M) [
39], in complex with Zn
2+ at several concentrations, and at different pHs (pH 7.5, 6.5, 5.5, 4.6), revealed three possible Zn
2+ binding sites (ZBS) [
40]. Crystals were grown by hanging-drop vapour-diffusion method in a solution composed of 100 mM sodium citrate, 2.0 ammonium sulphate and 200 mM of zinc acetate. The C-α r.m.s.d. among the structures (PDB id: 3DGD, 3GPS, 3GRB, 3GRG) is less than 0.4 Å, indicating that they are very similar to each other, a. The binding of Zn
2+ with ZBS1 involves the two amino acids Cys10 and His56 and it does not lead to any significant conformational change. This suggests that the allocation of Zn
2+ in ZBS1 is physiological and may prevent amyloidogenic character, b [
40].
Figure 3. TTR crystal structures in complex with Zn2+. The figure was created by author downloading the appropriate PDB codes. (a) Superposition of the four Zn2+-M-TTR obtained at different pHs (PDB id: 3GRG pH 7.5, 3GRB pH 6.5, 3GPS pH 5.5, 3DGD pH 4.6). Zinc ions are colored in grey. (b) Graphical representation of ZBS1-3 in the M-TTR crystal structure (PDB id: 3GRG). (c) Graphical representation of ZBS1-2 in the L55P-TTR crystal structure (PDB id: 3SSG).
Moreover, the occupation of ZBS1 does not produce any effect on TTR-RBP interaction (the residues of TTR involved in the binding with RBP are Arg21, Val20, Leu82 and Ile-84) [
41]. In contrast, in the presence of a higher Zn
2+ concentration, the binding of Zn
2+ to ZBS2 (Hys88, Hys90 and Glu92) and ZBS3 (Glu72, Asp74 and Hys31) b induces slight structural rearrangements around the α-helix at all tested pH. These modifications are comparable with those detected in other TTR structures crystallized without metals but at acidic pH [
42]. Contrary to the effect observed for ZBS1, the involvement of ZBS2 and ZBS3 and their corresponding conformational changes affect the interaction of TTR with RBP [
40].
TTR L55P is considered as one of the most aggressive amyloidogenic variants that accelerate pathology onset. Structural studies report that apo-TTR L55P, as well as TTR L55P in complex with 2,4-dinitrolphenol (DNP), possess the typical tetrameric structure. The only detected change is local in the monomers, where, due to a disorder of the short edge strand D, an extended loop between strands C and E appears [
43,
44].
The crystal structure of TTR L55P (PDB id: 3SSG), in complex with Zn
2+, does not show significant differences compared to apo-TTR, TTR L55P-DNP and the M-TTR-Zn
2+ [
45]. TTR L55P, in complex with Zn
2+, was grown by hanging-drop vapour-diffusion in presence of 3% w/v PEG 8000, 0.1M cacodylate pH 6.5, 5 mM Zn acetate. This X-ray structure shows two ZBS: ZBS1 is at an intra-dimer site that involves His90 from one monomer and Glu92 from the vicinal monomer, whilst ZBS2 is intra-tetramer, located between His31 and Asp74 from one monomer and Glu62 from symmetric monomer, c. No Zn
2+ ions are detected around Cys10 and/or His56; this might be related to the proximity of the point mutation L55P. Even if TTR L55P-Zn
2+ does not show relevant conformational changes, it is interesting to highlight that Zn
2+ binding induces a different tetragonal packing (space group P4
22
12) in the quaternary structure that could represent an ordered intermediate before evolving into an amyloidogenic conformation.
Recently, studies report that TTR can also be considered as an inducible metallopeptidase [
38,
46]. The TTR catalytic triad is composed of residues His88, His90 and Glu92, and its activation is modulated by bivalent metal ions. It has been demonstrated that, when TTR proteolytic activity is inhibited in vitro by metal chelators, Zn
2+ and Mn
2+ reestablished their full proteolytic activity, whereas other metals such as Fe
2+ and Co
2+ only partially reactivated the enzyme. This TTR proteolytic activity agrees with the hypotheses in which TTR behaves as a protease in neurodegenerative diseases such as AD and atherosclerosis. In fact, apoA-I and Aß can be cleaved by TTR, which might affect the onset of atherosclerosis and AD, respectively [
47,
48].
Despite several studies focusing on understanding whether the interaction between TTR and Zn2+ has a physiological or pathological role, the hypothesis is still debated.
2.2.2. Cu2+, Fe2+ and Mn2+
Biophysical studies employing light scattering and fluorescence spectroscopy demonstrate that Cu
2+ binds to TTR; the presence of the metal induces some structural and functional effects on TTR [
28]. Binding of Cu
2+ provokes a dose-dependent decrease in Trp41 fluorescence intensity, suggesting a local perturbation around this zone. The same tendency was observed when Cu
2+ binds TTR in the presence of 1-anilino-8-naphthalene sulfonate (ANS). ANS is a small fluorescent ligand able to bind both T4 binding sites and stabilize the TTR tetrameric structure [
49,
50]. The observation of a fluorescence perturbation upon Cu
2+ binding suggests a local structural variation across the central channel [
28]. Interestingly, the same study demonstrated that Cu
2+ did not influence the rate of wt-TTR amyloid formation at pH 6.5 or 7.4. This trend was successively confirmed in a urea-induced dissociation experiment. Depending on the tested concentrations, Cu
2+ did not show any effect on the tetramer dissociation, but, on the contrary, seemed to stabilize it. In contrast, in the L55P TTR mutant, Cu
2+ favors tetramer dissociation and accelerates the process of amyloid formation [
28].
As mentioned in the introduction, in contrast with its intrinsic amyloidogenic potential, TTR has a neuroprotective role in AD. TTR binds Aβ, participating in its clearance from the brain [
6,
51]. It has been hypothesized that metals might play a role in triggering this additional function of TTR.
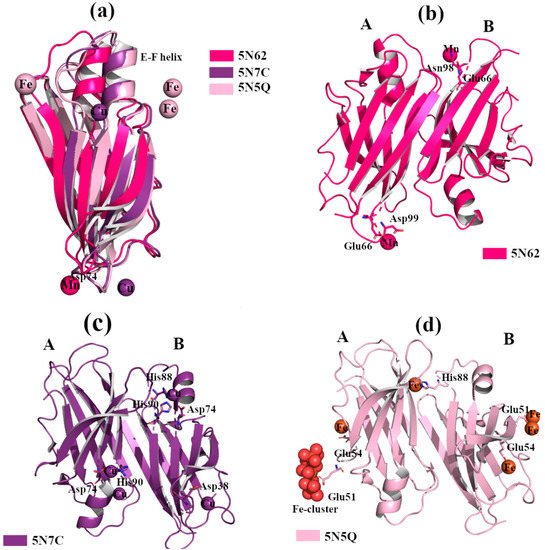
Structural studies of TTR in complex with different metals confirm that Fe
2+, Cu
2+ and Mn
2+ bind to the protein, a. TTR crystals were grown by sitting-drop vapor-diffusion, and the reservoir solution was filled with 21% of PEG4K, 0.14M imidazole malate, pH 6.0 or 21% of PEG4K, 0.14M imidazole malate, pH 6.0, 3.6% MPEG5K, and 30mM sodium acetate, pH 5.5. The cryoprotectant solution was composed of 40% of SM2 (12.5% ethylene glycol, 12.5% glycerol, 12.5% 1,2-propanediol, 25% DMSO and 37.5% 1,4-dioxane) 25% PEG 8K and 30mM of CuCl
2, MnCl
2 or FeCl
2. The X-ray crystal structure analysis of TTR in complex with Mn
2+ did not show any significant structural differences. Two possible Mn
2+ binding sites were detected around Asp99 and Glu66 in monomer A, and two, Glu66 and Asn98, in monomer B, b [
52]. In contrast, when wt-TTR crystals were treated with Cu
2+, these displayed a similar behavior of those soaked with Fe
2+ (a) and Re
2+ [
31,
52]. The electron density map suggests that Cu
2+ ions are located around His88, His90 and Asp74 for monomer B, and between His90 and Asp74 for monomer A, c. A minor pick is detected close to Asp38, c.
Figure 4. TTR crystal structures in complex with Mn2+, Cu2+ and Fe2+. The figure was created by author downloading the appropriate PDB codes. (a) Superposition of the B monomers of the three TTR crystal structures obtained in presence of Mn2+, Cu2+ and Fe2+, respectively (PDB id 5N62, 5N7C and 5N5Q). The EF-helix of TTR-Mn2+ crystal complex shows the classical conformation, while it is shifted for the other two metals complexes. (b) Graphical representation of the dimer A-B of TTR in complex with Mn2+. (c) Graphical representation of the dimer A-B of TTR in complex with Cu2+. (d) Graphical representation of dimer A-B of TTR in complex with Fe2+.
Thus, Fe
2+ and Cu
2+, but not Mn
2+, induce a conformational change in the wt-TTR tetramer, comparable to that observed in TTR-Re
2+ crystal complex [
52]. In order to verify if trivalent metals ions also induce conformational changes, several wt-TTR crystals were soaked with Al
3+, Gd
3+ and Fe
3+ following the same protocol. No structural modifications were found.
The binding of Fe
2+ with TTR protein was confirmed via the strong anomalous signal registered in the phased anomalous difference Fourier map [
53,
54]. The highest peak is registered close to Glu51, a second site is located near Glu54 at the entrance of the T4 binding site, and another peak is detected around His88, d. As previously seen in the rhenium-TTR structure, the conformational change affects only the β-strands E and F, and the short α-helix connecting them located in monomers B and B’ [
52].
Interestingly, the conformational change, induced by Cu
2+ and Fe
2+, modifies the dimer-dimer interface involving the TTR amino acid sequence, which is implicated in the interaction with Aβ peptides [
35,
55]. It is known that in the brains of AD patients, the concentration of metals (in particular Cu, Fe and Zn) is altered, and the amount of Cu
2+ in Aβ plaque can reach 400 µM [
24]. A bio-layer interferometry (BLI) study in solution revealed that a binding affinity between TTR and Aβ1-28 peptide is in nanomolar range when in the presence of Cu
2+, thus hinting that the TTR conformational change induced by Cu
2+ and Fe
2+ might be associated with TTR’s ability to neutralize Aβ [
52]. This experimental evidence suggests that the conformational change induced by Cu
2+ and Fe
2+ is not a structural artefact due to the soaking technique, but is probably related to the neuroprotective role that TTR possesses in the brain. This new conformation of TTR has inspired the design of PROteolysis-Targeting Chimeras (PROTAC) compounds that can induce the “active TTR conformation” favoring both the stabilization of TTR tetramer and the Aβ scavenger [
56].
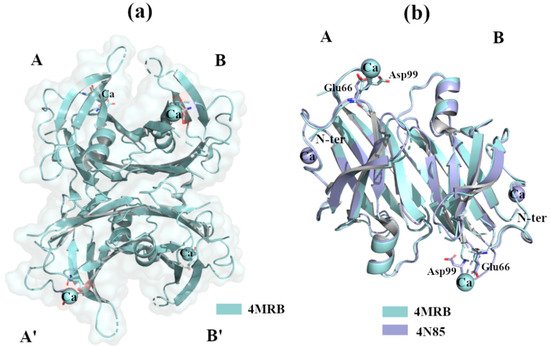
2.2.3. Ca2+
The calcium ion, Ca
2+, is one of the most important metals involved in the regulation of cellular signalling pathways and tissue homeostasis. Several studies report that the dysregulation of Ca
2+ is a key factor in the triggering of neurodegenerative processes [
57]. TTR binds Ca
2+ [
58], and X-ray studies do not show any relevant structural changes in the wt-TTR tetrameric structure. TTR-Ca
2+ crystal complexes were obtained by sitting drop vapour-diffusion from a solution containing 100mM HEPES, 200 mM CaCl
2, 28% PEG400, pH 7.5 (PDB id: 4MRB, 200 mM Ca
2+ [
59]) and 34–40% PEG 400, 400 mM CaCl
2, 0.1 M HEPES, pH 7.5 (4N85, 400 mM Ca
2+) [
60]. The superposition of the two structures reveals that there is a common Ca
2+ site between Glu66 and Asp99 of monomer A, while a second site is detected around N-terminus portions in the crystal at higher Ca
2+ concentrations, a,b. Different variants of TTR have been solved alone or in complex with ligands, in the presence of Ca
2+, and in these cases no relevant structural differences were detected [
59,
61,
62].
Figure 5. TTR crystal structures in complex with Ca2+. The figure was created by author downloading the appropriate PDB codes. (a) Tetramer representation of TTR in complex with Ca2+. (b) Superposition of the two dimers A-B of TTR in complex with Ca2+.
Recently, studies in solution show that Ca
2+ does not modify the environment around tryptophan residues, confirming that it does not induce global structural changes [
63]. Interestingly, in the presence of Ca
2+, the binding between TTR and ANS decreases, suggesting that the T4 binding sites are less accessible. Deeper analysis confirmed that the fluorescent emission spectra, recorded at 275 nm, did not display any significant structural modifications [
63]. However, the same study confirms that Ca
2+ increases the rate of fibril formation in the TTR fibril formation assay. This suggests that the dysregulation of Ca
2+ ions might have a role in the onset of TTR amyloidosis.