Aspartic acid, or “aspartate,” is a non-essential, four carbon amino acid produced and used by the body in two enantiomeric forms: L-aspartic acid and D-aspartic acid. The L-configuration of amino acids is the dominant form used in protein synthesis; thus, L-aspartic acid is by far the more common configuration. However, D-aspartic acid is one of only two known D-amino acids biosynthesized by eukaryotes. While L-aspartic acid is used in protein biosynthesis and neurotransmission, D-aspartic acid is associated with neurogenesis and the endocrine system. Aspartic acid production and use has been growing in recent years.
- bio-based
- bio-chemicals
1. Industrial Utility
2. Global Markets
3. Production and Manufacturing
| Category | Summary |
|---|---|
| Industrial importance and potential of biochemical | Aspartic acid is used in the food, beverage, pharmaceutical, cosmetic, and agricultural industries. The global aspartic acid market is projected to reach $101 million with a market demand of 60.6 kilotons by 2022 representing a compound annual growth rate of 5.6% [6]. |
| Industrial uses for biochemical | Aspartic acid is used in the production of: nutritional (amino acid) supplements; artificial sweetener (aspartame); polyaspartic acid hydrogels; and acetyl aspartic acid, the active ingredient in anti-aging cosmetics. |
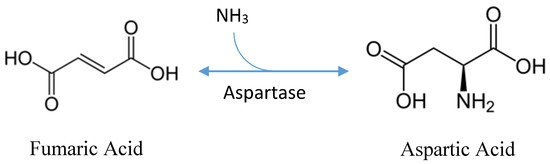
| Substrates used for the production of biochemical | primary substrate: fumaric acid [7] cofactor: ammonia [7] enzyme: L-aspartate ammonia-lyase [7] |
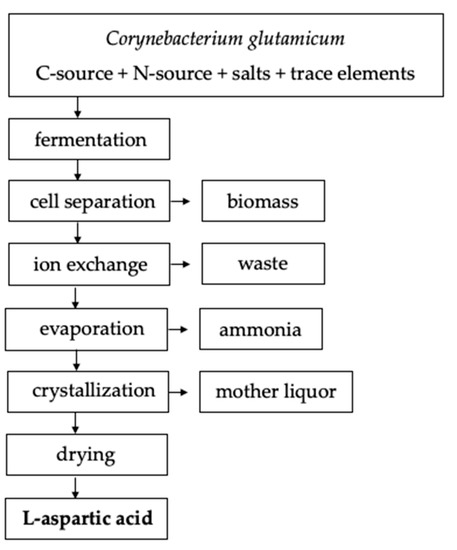
| Microorganisms used for fermentation | Primary industrial species:E. coli and Cornybacterium glutamacium [9] Exploratory species [1]: Pseudomonas aeroginosa Pseudomonas fluorescens Candida hydrocarbofumarica Bacillus stearothermophilus Bacillus subtilis |
| What enzymes are needed to break down the substrate for fermentation | Fumaric acid used in aspartic acid production does not need to be broken down, rather, it is fermentatively produced from glucose or chemically produced from maleic anhydride [6]. |
| Fermentation conditions used: pH, substrate loading, temperatures, times, maximum yield, maximum fermentation rates | pH is initialized to 7.0 [10] substrate concentration: 1:1 or 1:2 ammonia to fumaric acid [10] time 2 to 10 days [10] temperature 27–40 °C [10] yield 77–95% (w/w of fumaric acid) depending on bacterial strain and fermentation conditions [10] |
| Separation equipment, conditions, efficiencies | batch fermentation: separation via anion exchange column and crystallization [10] continuous fermentation: separation via isoelectric point precipitation and crystallization [10] |
| Total energy used to produce this chemical | Data not currently published. |
| Estimated costs to produce this chemical | Cost as well as upstream and downstream raw materials and equipment analysis available in the global L-aspartic acid market report provided by Market Watch (2019), at https://www.researchreportsworld.com/purchase/14314090 (accessed on 21 February 2021) |
| Current aspartic acid manufacturers | The following companies are the top industrial producers of aspartic acid [11]; the corresponding links, when applicable, are to each respective company’s product information page. Ajinomoto Group https://www.ajiaminoacids.com/product/l-aspartic-acid (accessed on 21 February 2021) Evonik https://healthcare.evonik.com/product/health-care/en/products/pharmaceutical-amino-acids/REXIM/pages/parenteralnutrition.aspx?xd_co_f=M2Q2OWQ5N2ItYTZkOC00ZWZjLThjNmUtODFiYjQ3YmYwM2I2 (accessed on 21 February 2021) KYOWA http://www.kyowahakko-bio.co.jp/english/products/aminoacids/l_aspartic_acid/ (accessed on 21 February 2021) Jinghai Amino Acid http://en.chinaaminoacid.com/products/L-AsparticAcid.shtml (accessed on 21 February 2021) JIRONG PHARM Not currently available OR product catalogue not in English Siwei Amino AcidEnglish product description not available Zhangjiagangxingyu Technology http://www.zjgxykj.com/template/p13e.html (accessed on 21 February 2021) Hubei Bafeng Pharmaceutical Company page not accessible in English |
| Potential market segments, sales, etc. | The aspartic acid market is segmented into six market categories: Feed Supplements, Medicine, Polyaspartic Acid, Aspartame, L-Alanine, and “Others” [6]. The report summary states that polyaspartic acid represents 22.6% of the total market volume in 2014. Market volumes and revenue values available upon report purchase [11]. |
| Primary economic setbacks and challenges | Fermentative production competes economically with petroleum-derived production. Economic setbacks of aspartic acid include high fumaric substrate cost and the low yields currently achieved by switching to cheaper sugar-based feedstocks [10]. Crystallization utilized in downstream processing separations can be expensive and time-consuming [1]. |
| Technological setbacks and challenges | The fermentative production of aspartic acid from glucose or sugar-based feedstocks, both much cheaper and more available substrates than fumarate, currently generate much poorer yields, i.e., 95% versus 29% [8]. Thus, the main technological setback to more economical aspartic acid production is the ability to directly ferment sugar to L-aspartic acid. |
| Side products, byproducts, waste products and associated cost | Organisms whose genomes also code fumarase (e.g., C. glutamicum, E. coli) produce malic acid from fumarate as a byproduct in effect utilizing substrate and decreasing aspartic acid yield. Without heat treatments Tajima et al. (2015) lost 25% of the fumaric acid substrate to malic acid production which translates to significant yield losses [7]. |
| Downstream processing operations | L-aspartic acid can be separated from the culture broth or eluate in batch systems via ion exchange resins utilizing an anion exchange column followed by crystallization of the eluate [1]. Continuous systems can extract the L-aspartic acid via isoelectric point precipitation (adjust broth pH to 2.8) followed by crystallization [10]. |
| New technologies, strains, equipment developments | Membrane reactor systems (MRS), as they are currently being developed, utilize growth-arrested cells eliminating the need for cell or enzyme immobilization [8]. The MRS system employed by Yukawa et al. (2009) overcomes the low mass transfer rates and low volumetric productivity issues associated with immobilization systems and simplifies the overall production process, allowing for easier separation of cells from the reaction mixture and generating high yield and productivity during long periods of operation [8]. Genetic modification of the metabolic pathways and feedback regulators within E. coli and C. glutamicum, the two major strains involved in industrial amino acid synthesis, are the next steps in improving L-aspartic acid production via the development of new, high-producing strains. |
| Yukawa et al. (2009) [8] | Tajima et al. (2015) [7] | Chibata et al. (1986) [13] | Szymanska et al. (2011) [14] | Papierz et al. (2007) [15] | |
|---|---|---|---|---|---|
| Pretreatments and Conditions Used | Genetically modify C. glutamicum to overproduce maleate isomerase and aspartase | E. coli DH5 used to produce plasmid containing aspA gene which is inserted into S. livingstonensis; sonicate then heat treat cells | Entrap E. coli in polyacrylamide gel via polymerization reaction then break gel in 3–4 mm diameter granules; wash granules in water | Immobilize cells in chitosan gel; culture in FF medium for biomass cultivation (or other chemically defined media as outlined on pg. 2) | Cell membrane permeabilization activates cells prior to aspartic acid production; perfomed in activation medium (chemically defined pg. 2) at 37 °C for 48 h |
| Substrate Used | Maleate ammonium | fumarate-NH3 | 1 M ammonium fumarate used for aspartic acid production by immobilized aspartase but no mention if substrate changed in subsequent trials | ammonium fumarate | fumaric acid |
| Substrate Loadings | Specifics not published | 860 mM fumarate-NH3 solution (pH 9) | 417 mM/h ammonium fumarate used for aspartic acid production by immobilized aspartase no mention if substrate loading changed in subsequent trials | 150.0 g/L ammonium fumarate | 100g/L fumaric acid 1 g biomass into 10 mL production media |
| Enzymes Used | maleate isomerase, aspartase | Enzymes are generated intracellularly | intracellular aspartase | intracellular aspartase | intracellular aspartase |
| Enzyme Loadings | Specifics not published | Not applicable | Not applicable | Not applicable | Not applicable |
| Reaction Times | production is continuous | 1–2 h | enzyme activity observed after 24–48 h found in production media; however, production can expands weeks in a continuous reactor | >603 h (production can be continuous) | 18–30 h |
| Bioreactor Conditions | pH 8.5 Temp 30 °C |
Heat treatment prior to fermentation performed in water bath; optimal conditions were 50 °C for 15 min | intended for continuous production; pack cells in a column reactor | biocatalyst bed height to volume ratio = 3:1; liquid hour space velocity value was 5.2 (i.e., volume of feeding substrate passed per volume of catalyst in bioreactor per one hour) |
100 mL shake flasks |
| Microorganisms Used | C. glutamicum | S. livingstonensis | E. coli ATCC 11303 | Escherichia coli mutant strains B-715 and P1 | Escherichia coli mutant strain B-715 |
| Fermentation Conditions Used (Temp, pH, etc.) | pH 8.5 Temp 30 °C |
whole cell production set at 37 °C for 3 h | Temp 37 °C half-life of column was 120 days |
initial media pH 8.5 Temp 40 °C |
initial media pH 8.5 Temp 37 °C |
| Separation Technologies Used | ultrafiltration | centrifugation, supernatant separated by HPLC with RI detector and ion exclusion column | Specifics not published | HPLC | HPLC |
| Separation Conditions Used | Specifics not published | Eluate at 60 °C using 0.1% (v/v) phosphoric acid for mobile phase with 0.7 mL/ min. flow rate; quantify via derivatization with DNFB | Specifics not published | HPLC with 250–4 LichrospherTM 100RP-18 (Merck) column and Waters fluorescence detector | Deproteinize with methanol, centrifuge, then run on HPLC column set to a flow-rate of 1 mL/min, 22 °C, and 2100 PSI with mobile phase of 200 mL methanol and 800 mL 0.05 M sodium phosphate buffer |
| Biochemical Yields Achieved | “High yield and productivity” hints that it should be within >95% range as achieved by immobilized cell methods; however, specifics not published | 95.2–99.3% | Immobilized aspartase had 29% activity yield but this “activity yield and the stability of the immobilized enzyme were not satisfactory for industrial purposes” thus the need to increase yield from this starting point in subsequent trials; for the set of conditions listed here, the results only mention that activity was notably increased | 99.8% conversion rate 6 g L-aspartic acid/g of cells /hour |
0.19–0.35 g L-aspartic acid/g of dry biomass/min during 1 h of biosynthesis |
| Inhibitory Compounds Observed, Developments and Impacts | L-malic acid is a byproduct (reduces yield) which can be avoided by inactivating fumarase via incubation at 45 °C for 5 h | L-malic acid also major byproduct (reduces yield) | Increased membrane permeability to substrate (“activation”) and later product increases enzyme activity and is the result of autolysis of the cells in the gel; Tween 80 required for E. coli P1 | Better immobilization and aspartic acid production with added surfactants for cell activation and a media 2-fold lower in yeast extract (found to be an inhibitory ingredient for biomass production) | Improved production following incubation in the activation medium containing 5 g/L ammonium fumarate |
| Notes | Incredibly limited in method detail and results | Exact methodology published, even greater detail in literature since multiple fermentation conditions were tested. | Review is very dated (1986); however, it covers several additional methods utilizing different gels and optimized parameters of base method. It appears to contain foundational work from which the popular immobilization technique of aspartic acid production was developed. | Highly detailed methodology | L-aspartic acid production was used to determine best aspartase-active mutant strain, i.e., conditions may not reflect requirements for scaled-up industrial production |
This entry is adapted from the peer-reviewed paper 10.3390/fermentation7020049
