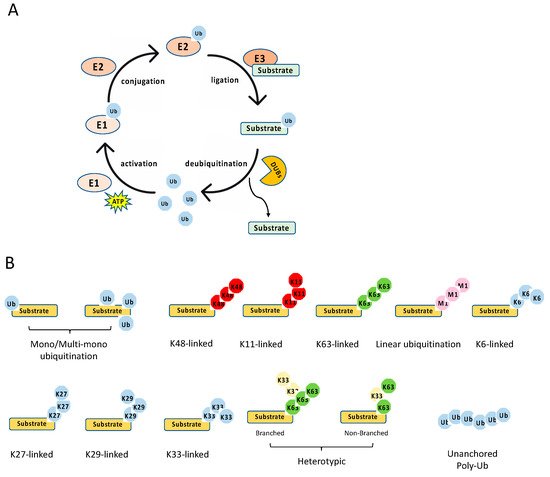
As mentioned above, many stimuli activate NF-κB pathways and the signal cascades upstream of IKK and NIK are diverse and complex, although they are also regulated by ubiquitination. Thus, this review only focuses on two examples, TNFR1 and RIG-I signaling pathways (). TNFR1 is a well-studied inflammatory cytokines-induced signaling pathway, while RIG-I is the major antiviral innate immune pathway in response to RNA virus infection. These pathways are not only critical for inflammation and host defense during viral infection but also are extensively examined for polyubiquitin-mediated regulation.
3.1. Ubiquitination in the TNFR1 Signaling Pathway
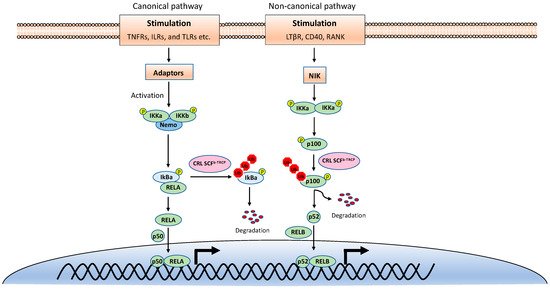
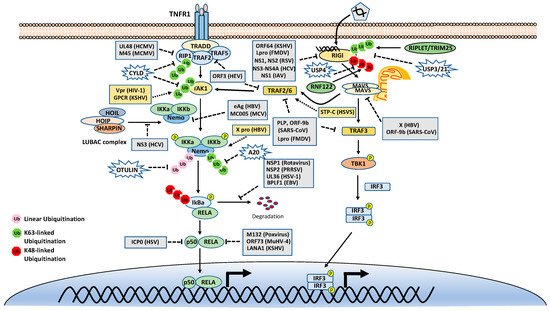
The TNF receptor 1 (TNFR1)-signaling pathway is the most well-studied canonical NF-κB pathway. TNFR1 is a receptor for the inflammatory cytokine, tumor necrosis factor-alpha (TNFα), which induces inflammation, apoptosis, and necrosis. Once engaged with TNFα, TNFR1 trimerizes and recruits different cellular adaptors to form complex I and complex II, leading to NF-κB activation and cell death, respectively. To fit the review topic, we focus on the signaling pathway elicited by TNFR1 complex I. Within the complex I, the tumor necrosis factor receptor type 1-associated death domain protein (TRADD) binds TNFR1 through the death domain. TRADD further recruits the receptor-interacting serine/threonine–protein kinase 1 (RIPK1) and the E3 ligases TNF receptor-associated factor 2 (TRAF2), TRAF5, and c-IAPs. cIAPs or TRAFs generate K63-linked polyubiquitin chains on TRAF2 and RIP1. The K63-linked polyubiquitin chains then recruit the TAK1-TAB complex and the IKK complex to the receptor complexes via K63-selective binding of TAB2/3 or NEMO, respectively. Recent studies also showed that the LUBAC E3 ligase complex is also recruited to the TNFR1 and generates Met1-linked polyubiquitin on RIP1 and NEMO [
26]. These Met1-linked polyubiquitin chains also function as a scaffold to recruit the IKK complex via the ubiquitin-binding domain of NEMO. Within the TNFR1 signalosome complex, TAK1 activates the IKK by phosphorylation, and then the activated IKK complex phosphorylates IκBα. The phosphorylated IκBα is ubiquitinated by the E3 complex SCFβ-TrCP for the K48-ubiquitination-mediated proteasomal degradation of IκBα. After liberation from IκBα, the canonical NF-κB transcription factors, predominantly composed of homo- or hetero-dimers of p65 (RelA) and p50, translocate into the nucleus and activate NF-κB target genes ().
DUBs are also engaged with a opposite role to E3 ligases by balancing ubiquitination modification. The K63-linked polyubiquitin is hydrolyzed by the cylindromatosis gene CYLD and A20 (also known as TNFα-induced protein 3). CYLD contains a USP domain in the C-terminus, which mediates the cleavage of polyubiquitins. In the NF-κB pathways, CYLD removes the K63-linked polyubiquitin chains from TAK1, RIP1, BCL3, TRAF2, TRAF6, and NEMO. Unlike CYLD, A20 is a hybrid of DUB and E3 ligase, which has an N-terminal OTU domain responsible for polyubiquitin cleavage and a C-terminal domain of seven Cys2-Cys2 zinc-fingers (ZF) that render the E3 ligase activity. A20 disassembles K63-linked polyubiquitin chains of RIP1, TRAF6, RIPK2, NEMO and MALT1, thus inhibiting the activation of NF-κB activation. A20 also mediates K48-linked ubiquitination of RIP1, leading to its degradation, thus inhibits NF-κB activation. The cellular zinc finger anti-NF-κB (Cezanne) is a deubiquitinase of the ovarian tumor superfamily with sequence similarity to A20. Cezanne also suppresses NF-κB activation by targeting RIP1 signaling intermediaries for deubiquitination [
36].
In addition, the ubiquitin-specific peptidase 21 (USP21) inhibits NF-κB in the TNF pathway by deubiquitination of RIP1 [
37]. USP31 and USP7 also inhibit NF-κB signaling by deubiquitinating K63-linked polyubiquitin chains [
38,
39]. MCP-induced protein 1 (MCPIP1) (also known as Zc3h12a) deubiquitinates TRAF proteins and negatively regulates JNK and NF-κB signaling [
40]. USP11 and USP15 remove K48-linked chains from IκBα, thus protecting IκBα from degradation by the proteasome [
19,
20]. Interestingly, USP2 positively regulates TNF-induced NF-κB activation; however, the target of USP2 is unknown [
41].
The Met1-linked polyubiquitin chain is cleaved by CYLD and OTULIN. CYLD exhibits deubiquitinase activity toward multiple types of polyubiquitin; however, OTULIN specifically targets Met1-linked polyubiquitin. OTULIN also interacts with HOIP via its PUB-interacting motif and inhibits LUBAC activity [
42,
43]. Recently, we found that TRIM32 conjugates nonproteolytic polyubiquitin onto OTULIN and the polyubiquitin blocks the interaction between HOIP and OTULIN, thereby activating NF-κB signaling [
44].
3.2. Ubiquitination in the RIG-I Signaling Pathway
The innate immune system uses pattern recognition receptors (PRRs) in different cellular compartments to sense microbial components that mark invading viruses. Many of these PRRs have been characterized, including the RIG-I-like receptors (RLRs), such as RIG-I, MDA5 and LGP2 [
45,
46]. The RLRs recognize viral RNA in the cytoplasm, for example, the double-stranded RNA (dsRNA) or 5’ triphosphate RNA generated by viral replication of RNA viruses [
47,
48,
49,
50,
51,
52]. The engagement of viral RNA induces the conformational change of RIG-I and several post-translational modifications, which leads to the oligomerization of RIG-I. The oligomerized RIG-I translocates to the mitochondria and binds the mitochondrial antiviral signaling protein (MAVS, also known as CARDIF, IPS1, and VISA). The binding results in the oligomerization of MAVS and the recruitment of TANK-binding kinase 1 (TBK1). The oligomerized MAVS further recruits TBK1, TRAF6, IKK and interferon regulatory factor 3 (IRF3). Subsequently, TBK1 phosphorylates IRF3, which in turn triggers its dimerization and nuclear translocation [
53,
54,
55,
56,
57], whereas IKK phosphorylates IκBα to release NF-κB. In the nucleus, IRF3 and NF-κB, together with other transcription factors, form active transcriptional complexes and activate type I IFN expression [
53,
54,
55,
56,
58] ().
Similar to the TNFR1 signaling pathway, the RIG-I signaling pathway is also heavily regulated by ubiquitination. First, RIG-I undergoes several types of ubiquitination, which is regulated by at least nine E3 ligases and four DUBs. Three ubiquitin E3 ligases, TRIM25 [
59], MEX3C [
60], and TRIM4 [
61], have been reported to activate RIG-I signaling by mediating K63-linked polyubiquitination of the N-terminal CARD domain of RIG-I. TRIM25 was first found to bind the CARD and mediate polyubiquitination of CARD at Lys172 [
59]. Other studies showed that MEX3C and TRIM4 target RIG-I for ubiquitination at different sites [
60,
61]. Recently, a study showed that another ubiquitin E3 ligase, RIPLET (a.k.a. REUL), is the predominant E3 ligase for RIG-I K63-linked ubiquitination and activation [
62,
63,
64]; however, RIPLET ubiquitinates the CTD domain of RIG-I at Lys849 and Lys851 [
65]. Interestingly, unanchored K63-linked polyubiquitin is also found to non-covalently bind the CARD and promote CARD tetramerization and concomitant signal activation [
66]. These activating K63-linked polyubiquitin chains of RIG-I can be removed by several DUBs, including USP3, USP21 and CYLD [
67,
68,
69,
70]. RIG-I also can be conjugated with K48-linked polyubiquitin. The E3 ligases RNF122, RNF125, c-Cbl, and CHIP have been found to mediate K48-linked ubiquitination and proteasomal degradation of RIG-I, thereby inhibiting RIG-I signaling [
71,
72,
73,
74]. Another E3 ligase TRIM40 conjugates both K27- and K48-linked polyubiquitin onto RIG-I for proteasomal degradation [
75]. The K48-linked ubiquitination of RIG-I is reversed by USP4 [
76]. In addition, LUBAC mediates K48-linked ubiquitination of TRIM25, an E3 ligase for RIG-I K63-linked ubiquitination and activation. The K48-linked ubiquitination leads to TRIM25 protein degradation, thereby inhibiting RIG-I activation [
33]. In contrast, USP15 deubiquitylates TRIM25, preventing the LUBAC-dependent degradation of TRIM25 [
77].
The downstream of RIG-I is also regulated by ubiquitination. As with RIG-I, MAVS is also ubiquitinated multiple E3 ligases, although most of them mediate K48-linked ubiquitination and proteasomal degradation. Two HEC domain-containing E3 ligases, the Smad ubiquitin regulatory factor 1 (Smurf1) and Smurf2, also mediate K48-linked ubiquitination of MAVS [
78,
79]. Other E3 ligases, including MARCH5, RNF5, AIP4, TRIM25, and pVHL, are all reported to promote MAVS protein degradation through K48-linked polyubiquitination [
80,
81,
82,
83]. Unlike the redundancy of E3 ligases for K48-linked polyubiquitination, there is only one E3 ligase TRIM31 was reported to confer the K63-linked polyubiquitin onto MAVS and promotes MAVS aggregation and activation [
84]. TRIM21 promotes innate immune response to RNA viral infection through K27-linked polyubiquitination of MAVS [
85]. There are two DUBs for MAVS, the ovarian tumor family deubiquitinase 4 (OTUD4) and YOD1, cleaves K48- and K63-linked polyubiquitin chains of MAVS, respectively [
86,
87]. Interestingly, TRIM44, an unusual DUB, stabilizes MAVS by preventing its ubiquitination and degradation [
88]. In addition, K63-linked ubiquitination of TRIM14 bridges NEMO to MAVS [
89].
Interestingly, two E3 ligases catalyze non-K48-linked polyubiquitin chains on MAVS to promote its autophagic degradation. The E3 ubiquitin ligase MARCH8 catalyzes the K27-linked polyubiquitin chain on MAVS at lysine 7, which serves as a recognition signal for NDP52-dependent autophagic degradation [
90]. Another E3 ligase RNF34 initiates the K63- to K27-linked polyubiquitin switch on MAVS at Lys 311, thus facilitating the autophagic degradation of MAVS [
91].
TBK1 is the key kinase for the IRF3 signaling axis of the RIG-I pathway. We and others showed that TBK1 is K63-linked ubiquitinated for activation by E3 ligases mind bomb 1 (MIB1), MIB2, and RNF128 [
92,
93]. Overall, the RIG-I signaling pathway is heavily regulated by different types of ubiquitination, which provides multiple layers of regulation for this pathway.