Gastric and esophageal tumors are diverse neoplasms that involve mucosal and submucosal tissue layers and include squamous cell carcinomas, adenocarcinomas, spindle cell neoplasms, neuroendocrine tumors, marginal B cell lymphomas, along with less common tumors. The worldwide burden of esophageal and gastric malignancies is significant, with esophageal and gastric cancer representing the ninth and fifth most common cancers, respectively. The approach to diagnosis and staging of these lesions is multimodal and includes a combination of gastrointestinal endoscopy, endoscopic ultrasound, and cross-sectional imaging. Likewise, therapy is multidisciplinary and combines therapeutic endoscopy, surgery, radiotherapy, and systemic chemotherapeutic tools. Future directions for diagnosis of esophageal and gastric malignancies are evolving rapidly and will involve advances in endoscopic and endosonographic techniques including tethered capsules, optical coherence tomography, along with targeted cytologic and serological analyses.
- esophageal cancer
- gastric cancer
- gastrointestinal stromal tumor
- neuroendocrine tumor
- MALT lymphoma
- mucosal resection
- submucosal dissection
1. Introduction
1.1. Anatomic Principles
Upper gastrointestinal neoplasia is a complex disease process of the digestive organs of the foregut involving structures from the upper esophageal sphincter to the duodenum at the ligament of Treitz. These lesions originate from the mucosal or submucosal tissue layers of the esophagus, stomach, and duodenum and involve numerous cell types, including squamous cell carcinomas, adenocarcinomas, spindle-cell neoplasms, lymphomas, neuroendocrine tumors, and several less common tumors.
1.2. Epidemiology
Available WHO statistics indicate that upper gastrointestinal malignancies are responsible for a significant disease burden globally. Esophageal cancer (including adenocarcinoma and squamous cell carcinoma) is the ninth most common malignancy worldwide and has the sixth-highest cancer mortality [1]. Squamous cell cancer of the esophagus accounts for approximately 90% of incident esophageal cancers all over the world. It is much less common than adenocarcinoma in the United States. Gastric cancer (adenocarcinoma) represents an even greater disease burden, and is the fifth most common cancer, representing the fourth-highest cause of cancer mortality worldwide [2]. Gastric adenocarcinoma exhibits a unique geographic predilection, with a high incidence documented in Asian countries, particularly in Japan and South Korea (41 cases per 100,000 persons) and lowest in North America (below 5 cases per 100,000 persons) [3]. Gastric adenocarcinoma is subdivided into the intestinal and diffuse subgroups based on the Lauren classification, which includes intestinal, diffuse, mixed, and indeterminate histological variants. Alternatively, the WHO classification is based upon histology and subdivides gastric adenocarcinoma into tubular, papillary, mucinous, poorly cohesive, and rare variants [2][4].
Gastric lymphomas account for 1–6% of gastric neoplasms diagnosed annually. Infection with H. pylori is known to be an important factor in carcinogenesis. Chronic H. pylori infection is thought to promote clonal expansion of gastric lymphocytes, leading to gastric lymphoma. Approximately 40–50% of cases are gastric marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT) and 20–40% are extra-nodal lymphomas [5].
Gastrointestinal stromal tumors (GISTs) are rare mesenchymal tumors. They may occur anywhere along the digestive tract, but they are most commonly located in the stomach and small intestine. Within the US, the incidence is estimated at 4000 to 6000 cases per year [6].
Gastrointestinal neuroendocrine tumors (NET) are uncommon and may occur anywhere along the gastrointestinal tract or within the pancreaticobiliary system. There are four subtypes of gastric neuroendocrine tumors (I, II, III, and IV). Type I tumors are associated with atrophic chronic gastritis. They may be well-differentiated and multifocal. Smaller type I lesions may be amenable to endoscopic resection and surveillance. Type II NETs are associated with Zollinger Ellison Syndrome (as part of Multiple Endocrine Neoplasia I) and have an increased malignant potential. Type III and Type IV neuroendocrine tumors have a higher malignant potential and may be metastatic on presentation. In the US, from 2000 to 2012, SEER 18 Registry data indicated a neuroendocrine tumor incidence of 3.56 per 100,000 in gastroenteropancreatic sites. It is estimated that only 7–8% of NETS are found in the stomach [6].
1.3. Natural History
Adenocarcinomas, squamous cell carcinomas, neuroendocrine tumors, and spindle-cell neoplasms of the upper GI tract each demonstrate a characteristic natural history. The therapeutic approach is determined by numerous factors. These factors include tissue of origin, histology, anatomic location, size, and anatomic stage. It is important to consider precursor conditions (e.g., Barrett’s esophagus, intestinal metaplasia) in the approach to surveillance, diagnosis, and ultimately therapy of upper GI malignancies.
Barrett’s esophagus is a premalignant condition characterized by specialized intestinal metaplasia (SIM) within the distal esophagus. It is considered a precursor lesion in the development of esophageal adenocarcinoma and is related to numerous factors, including chronic reflux of gastric acid and bile salts into the lower esophagus [7][8]. Similarly, intestinal metaplasia within the stomach can lead to the development of intestinal-type gastric adenocarcinoma over time. Risk factors include chronic bile reflux, high dietary salt intake, smoking, alcohol consumption, and consumption of nitrates or smoked foods [9]. H. pylori gastritis is also an important risk factor in the development of gastric intestinal metaplasia, with some studies showing that individuals with associated H. pylori infection have a six-fold increase in developing gastric cancer [10][11].
1.4. Approaches to Diagnosis, Staging, and Therapy
Endoscopy with high-definition white light imaging is recommended and is the standard for detecting and documenting the presence of mucosal or submucosal lesions, as shown in Figure 1. Non-invasive approaches to screening for premalignant lesions of the esophagus include devices such as the “EsophaCap” [12] and cytosponge -TFF3 that detect genetic and epigenetic alterations on samples gathered non-invasively and can be used by primary care physicians to screen for Barrett’s esophagus [13]. Non-invasive diagnostic modalities, using molecular biomarkers from a variety of body fluids to diagnose early gastric cancer, have been developed. These include “liquid-based biopsy,” which uses circulating nucleic acids. This is an exciting area of research that may change the diagnostic landscape [14].
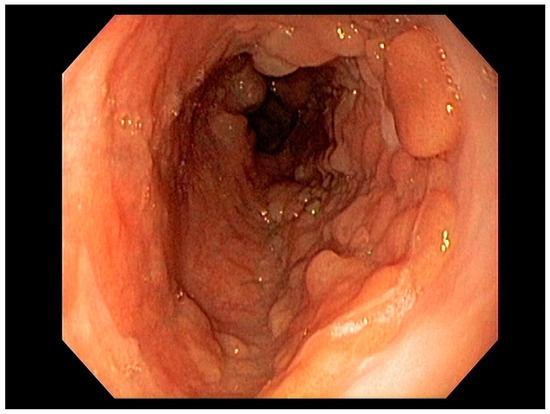
Figure 1. Barrett’s esophagus with Nodules White Light Endoscopy.
Early endoscopic detection of premalignant lesions such as Barrett’s esophagus, gastric intestinal metaplasia, and mucosal cancer using optical chromoendoscopy techniques such as narrow-band imaging (NBI) combined with high-definition white light imaging (HD-WLE) is becoming routine across the world. Targeted biopsies from endoscopically suspicious areas or using the updated Sydney protocol are important for successful diagnosis [15][16]. In the presence of dysplasia with visible lesions or carcinoma, endoscopic ultrasound (EUS), a minimally invasive procedure, has a well-established role in the early diagnosis and locoregional staging of non-metastatic foregut malignancies [17][18][19], see Figure 2.

Figure 2. Barrett’s esophagus with nodules under narrow band imaging (NBI) (arrows).
1.5. Endoscopic Approaches to Therapy
Factors including lesion size, histological features, anatomical stage, and esophageal location are important variables for determining favorability of endoscopic therapy, demonstrating curative resection in some studies [20]. Adenocarcinoma of the esophagus, limited to the mucosa (T1a)—see Figure 3—is amenable to endoscopic resection, with excellent long-term outcomes. Other tumors such as neuroendocrine tumors of the foregut may also be amenable to endoscopic therapy, including endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD). Specifically, these therapeutic approaches are used for resection of mucosal-based lesions, depending on the size, location, submucosal involvement, and complexity.
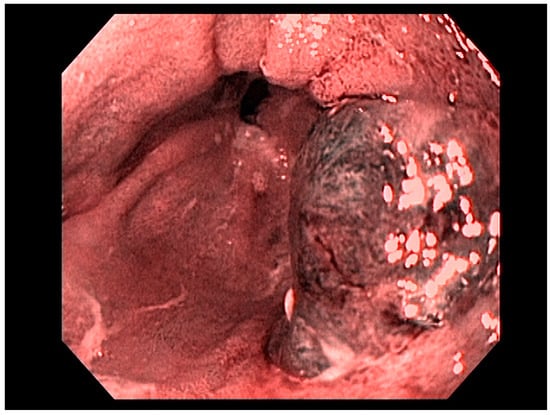
Figure 3. T1a esophageal cancer in Barrett’s Esophagus.
1.6. Role of Multidisciplinary Care
Treatment of upper gastrointestinal malignancies must be individualized and based on an interdisciplinary discussion in a tumor board setting, facilitated by National Comprehensive Cancer Network (NCCN) guidelines and institutional clinical pathways. In a Dutch study, the diagnostic or treatment plan was altered after a multidisciplinary tumor board discussion in over one third of cases [21]. A recent systematic review has endorsed the importance and impact of a multidisciplinary tumor board discussion in the diagnosis and management of patients with GI malignancies [22].
In addition to patient and clinical factors (e.g., functional status, comorbidities, and patient preferences), the therapeutic strategy is highly dependent on histological parameters (e.g., type of neoplasm, grade/differentiation, mitotic rate/Ki 67%, and other unique histological markers of response to certain therapies such as HER2/neu expression), anatomy/location, and, most importantly, the stage of the tumor [23][24]. The management approach may therefore involve a combination of chemotherapy, radiotherapy, endoscopic therapy, and surgery.
3. Treatment
3.1. Endoscopic Therapeutic Options for Barrett’s Esophagus Associated Dysplasia
Therapeutic options are based upon the stage of the disease at diagnosis.
Barrett’s esophagus with low- or high-grade dysplasia (HGD) without visible lesions can be treated with radio-frequency ablation (RFA) or cryoablation therapies [34][44]. A randomized, controlled trial demonstrated that (RFA) for Barrett’s esophagus with HGD was superior to surveillance with respect to the outcome of progression to esophageal adenocarcinoma but did not find any difference in progression to cancer among patients with low-grade dysplasia (LGD) [34]. A European, multicenter, randomized, controlled trial in a large group of patients with Barrett’s esophagus and LGD proved that RFA prevents progression to cancer in patients with LGD [45]. After RFA for BE dysplasia, there is >30% chance of recurrence within 5 years, but most recurrences are responsive to further endoscopic therapy [46].
Visible, discrete mucosal lesions within the Barrett’s segment, including high-grade dysplasia, T1a adenocarcinoma, and T1b adenocarcinoma with favorable histological features [47], can be approached with endoscopic resection techniques. EMR is a widely utilized endoscopic resection technique, performed using either the “Cap-assisted EMR (Cap-EMR)” technique or multiband mucosectomy (MBM) techniques with the primary goal of assessing the depth of invasion (pathological T-stage) of a visible lesion that is suspicious for intramucosal adenocarcinoma or high-grade dysplasia; see Figure 5. EMR and ESD are performed with intent of “staging” but can indeed be curative if the resection margins are “negative” or free of cancer, but the primary goal in Barrett’s esophagus is to obtain an accurate pathological T-stage of the lesion. This concept of “Staging-EMR” is demonstrated by studies that have shown a change in pathological diagnosis and/or T-staging noted in 20–30% of patients, both upstaging and downstaging, based on the pathology from the EMR specimen [48], see Figure 6.
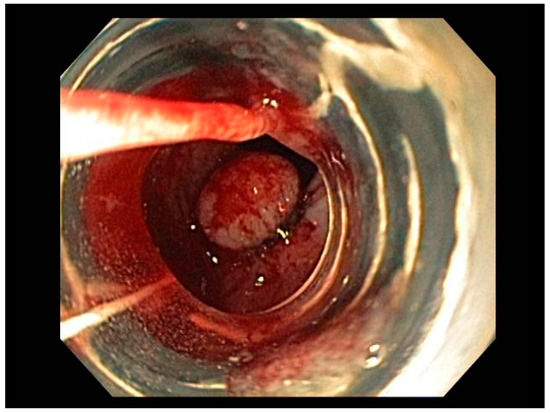
Figure 5. Band-assisted mucosectomy.
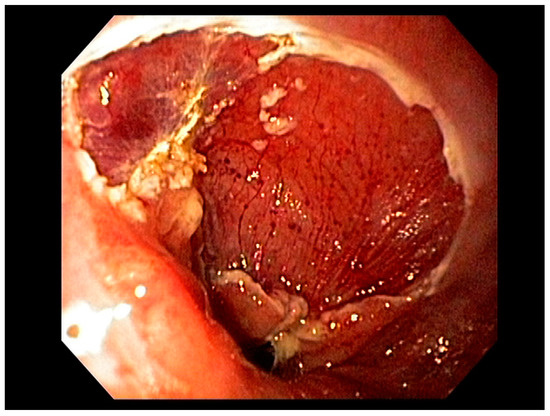
Figure 6. Endoscopic Mucosal Rejection (EMR) T1 esophageal cancer; resection site.
3.2. Endoscopic Resection Techniques for Early-Stage Esophageal Cancer (T1a and T1b)
Endoscopic resection techniques applied to T1a and T1b mucosal cancer with favorable histological features include EMR or ESD. As mentioned earlier, EMR is primarily a “staging procedure” that provides the most accurate T-stage for mucosal lesions that are likely T1 based on EUS and/or optical chromoendoscopy [49][50]. EMR is a technique that involves submucosal injection of saline or a colloidal solution mixed with a dye such as methylene blue to create a “submucosal cushion”, followed by resection using a snare, and may be considered for lesions less than 20 mm. Techniques such as Cap-EMR, MBM, and ESD may be employed depending on anatomical location [51][52].
Cap-assisted EMR involves submucosal injection to lift the target lesion from the mucosa, isolating the lesion within a transparent “cap” mounted on the tip of the endoscope, gentle suction, followed by resection of the lesion using the pre-deployed snare within the cap using electrocautery [53]. MBM involves suction of target tissue into an endoscopic band-ligation device and deployment of a band over the target lesion followed by resection of the tissue with electrocautery snaring [54]. EMR can be either stepwise/complete (sEMR) or focal (fEMR), with focal EMR often combined with RFA. Piecemeal EMR has a high local recurrence and therefore is combined with RFA to ablate the residual BE epithelium [55]. In theory, ESD is an en-bloc resection technique with the goal being an R0 resection and is therefore a more appealing endoscopic therapeutic option.
ESD is a more complex technique that may be considered for lesions less than 20 mm. Larger lesions may be amenable to this technique depending on availability of advanced expertise and resources [56]. Specifically, this technique involves the creation of a submucosal tract to dissect lesions restricted to the mucosa (T1a or selected T1b), for complete resection. Factors including lesion size less than approximately 20 mm, anatomical stage (T1a), and middle thoracic and lower thoracic esophageal locations are favorable variables for successful ESD, demonstrating curative resection in some studies [20][56]. Additionally, T1a m2, T1a m3, and select T1b lesions with favorable histological features [57] are amenable to ESD, with comparable outcomes to esophagectomy [58][59]. For these types of lesions, current evidence shows that EMR and ESD are comparable in terms of complication rates, referral to surgery, positive margins, lymph node positivity, local recurrence, and metachronous cancer. When compared to piecemeal EMR resection, ESD may offer some advantages, but data are limited [60][61]. Meta-analyses comparing both of these therapies revealed comparable outcomes in terms of Barrett’s eradication, low rates of recurrence, and have shown non-inferiority of EMR to ESD for therapy of Barrett’s related cancer or GEJ neoplasia, with similar complication rates [15][62].
3.3. Multimodality Therapy for Locally Advanced Esophageal Cancer
For locally advanced tumors (T2 or with nodal involvement), a combination of neoadjuvant chemoradiation therapy and surgical therapy can be used, with systemic chemotherapy being reserved for metastatic disease (Stage IV) with possible consolidation chemoradiation to sites of disease involvement if the patient responds well [63].
Based on current guidelines, esophagectomy for operable non-metastatic patients with T1b or greater primary lesions and/or any nodal disease should be performed with one of several techniques including: transhiatal, transthoracic, three field, and, increasingly, minimally invasive approaches [64]. Meta-analyses of minimally invasive esophagectomy, which includes laparoscopic/thoracoscopic and robotic approaches, have shown no difference in survival and improved or no difference in complications. Robotic-assisted esophagectomy is becoming increasingly popular, with demonstrated reduction in cardiac and pulmonary complications when compared with open esophagectomy. Technologic advances in the minimally invasive and robotic platforms are also rapidly developing and may allow for further innovation with these techniques [65][66]. While surgery alone can be considered for early-stage, low-risk adenocarcinoma (<3 cm and well differentiated) and early-stage squamous cell carcinoma, trimodality therapy with neoadjuvant chemoradiation followed by surgery is now a preferred treatment pathway for more advanced disease and requires multidisciplinary management [67]. Less commonly, postoperative chemoradiation for pathologic T3 and/or node positive disease may be indicated if a GEJ cancer was treated with preoperative chemotherapy, while palliative radiotherapy may be indicated for treatment of significant dysphagia or bleeding. In definitive treatment of inoperable patients or those who decline esophagectomy, concurrent chemoradiation should be prioritized, followed by imaging and endoscopic surveillance to determine clinical complete response [68]. While clinical outcomes and morbidity for these patients are still suboptimal, there are a growing number of long-term survivors after definitive chemoradiation and investigations are being made into radiotherapy dose escalation, proton therapy, and inclusion of targeted therapy [69][70][71][72].
Three-dimensional conformal planning for radiation therapy can be utilized; however, more institutions have implemented intensity-modulated radiation therapy (IMRT) to reduce cardiac and lung dosing, which allows for a simultaneous integrated boost (SIB) or sequential boost techniques [73][74][75]. This approach is important as cardiac dose is increasingly seen as an independent risk factor for reduced survival [76] and has been correlated with excess G3+ cardiac toxicity [77][78].
Fiducial markers can be placed endoscopically to delineate the extent of disease with high technical success with a small risk of migration [79][80][81]. Stable fiducial markers improve the reliability of target volume delineation and assessment of respiratory tumor motion with four-dimensional CT (4D-CT) simulation as a direct visual correlate of tumor extent. Combined with fused PET/CT, fiducials reduce the margins for treatment planning due to improved confidence of accurately defined gross tumor volumes (GTVs) [82] and facilitate daily image-guided radiation therapy (IGRT) during treatment [83], see Figure 7 and Figure 8. Moreover, 4D-CT imaging at simulation has a greater benefit in GEJ and gastric tumors due to the propensity of respiratory motion [84] and aids in internal target volume construction and planning target volume expansions during treatment planning. Radiation treatments generally are conventionally fractionated (1.8–2.0 Gy daily); however, previously mentioned SIB techniques treating at 2.2–2.25 Gy daily can push dose to gross disease past 60 Gy. If given sequential neoadjuvant chemotherapy, the extent of original disease is often included in the clinical target volume receiving 4500–5040 cGy and boost is only directed to residual gross disease. Clinical target volumes extend 3–4 cm craniocaudal and 1 cm radially and are edited off anatomical structures that are clinically uninvolved such as vertebral bodies, trachea, aorta, lung, and pericardium. For middle and upper thoracic tumors, the at-risk periesophageal and adjacent mediastinal lymph nodes and, for distal esophageal and GEJ tumors, the celiac lymph nodes are covered. Multi-institutional consensus contouring guidelines for IMRT in esophageal and gastroesophageal tumors have been published [85].
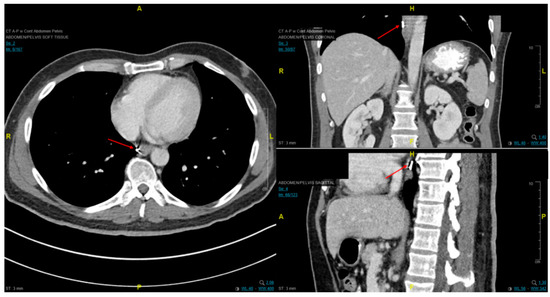
Figure 7. Axial, coronal, and sagittal CT images demonstrating EUS-placed fiducial (red arrow) proximal to esophageal tumor.
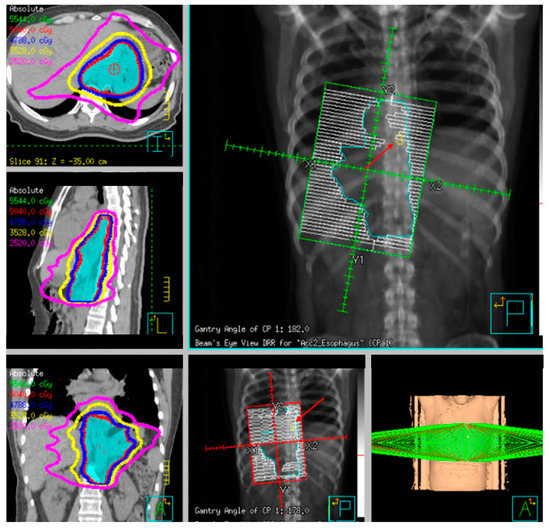
Figure 8. Example radiotherapy plan for image-guided treatment with fiducial contoured (red arrow) and within the planning target volume (PTV).
3.4. Neoadjuvant Radiation Therapy Alone and Adjuvant Therapy
Neoadjuvant treatment carries the theoretical gains of tumor downstaging, margin attenuation, and improved control of regional disease. However, limited benefit has been observed across several randomized trials investigating the role of radiotherapy alone in preoperative or adjuvant treatment of esophageal cancer. Early studies on patients who received 4000 cGy followed by surgery 1–4 weeks after found no difference in resectability or survival compared to surgery alone [86][87]. A trial from the European Organization for Research and Treatment of Cancer (EORTC) also demonstrated no difference in survival, but noted a lower rate of local failure with the addition of radiotherapy to 46% from 67% [88]. More recent studies that reported improved survival included patients that received chemotherapy but had an imbalance of lower stage tumors conflicting the interpretation of the results [89]. These findings have been confirmed in meta-analyses that demonstrate no statistical difference in survival, or at best, the suggestion of a very modest clinical benefit of <4% with neoadjuvant radiotherapy alone [90][91].
Recommendations for postoperative or adjuvant treatment are generally driven by adverse pathological features such as positive lymph nodes, positive margins, or locally advanced disease found at time of surgery. While the goal of local therapy is to reduce the risk of local recurrence, often, toxicity is greater due to larger treatment volumes, efficacy is reduced due to a hypoxic tumor bed, and dose is limited by the surgical anastomosis. In two historic series, adjuvant radiotherapy alone showed no survival benefit compared to observation [92][93]. However, similar to preoperative treatment, there are trends towards improved local control after delivery of 4500–5500 cGy [93] after subtotal resection [92]. Retrospective data and population-level analyses show a benefit of adjuvant chemoradiation in pathologically node positive [94] but not node negative disease [95]. The benefit of the addition of chemotherapy to adjuvant radiation is currently being investigated in a phase II/III protocol seeking to add paclitaxel and cisplatin/nedaplatin to 50.4–54.0 Gy of radiotherapy for pathological stage IIB-III disease [96].
3.5. Preoperative Chemoradiation
For locally advanced resectable disease, the CROSS trial (Chemoradiotherapy for Oesophageal Cancer Followed by Surgery Study) established a new paradigm towards total neoadjuvant treatment with chemoradiation followed by surgical resection in esophageal cancer [97]. Other comparisons to neoadjuvant chemotherapy alone in the German POET trial [98] and the NeoRes trial [99] demonstrated improvements, with pathological downstaging with the addition of 30 and 40 Gy of chemoradiation, but did not translate to statistically significant survival differences, likely limited by increased toxicity in the experimental arms with concurrent cisplatin/flurouracil. Within CROSS, radiation therapy was delivered to 4140 in 1.8 daily fractions with concurrent paclitaxel/carboplatin and was compared to surgery alone. Neoadjuvant chemoradiation was associated with a 25-month improvement in median overall survival (49 vs. 24 months) and was notable for a 47% 5-year survival rate and 29% overall pCR rate that was significantly different between histologies (28% adenocarcinoma vs. 49% squamous) [100]. Additionally, preoperative chemoradiation reduced hematogenous metastases (35% vs. 29%) and peritoneal carcinomatosis (14% vs. 4%). There was no difference in operative mortality between the arms or long-term quality of life, with all endpoints declining after surgery and subsequently improved and stabilized between 6 and 12 months afterwards [101].
The CROSS trial excluded patients with tumor diameter greater than 8 cm and patients who had lost more than 10% of their original body weight. In these patients, upfront chemotherapy alone can be considered as they are at high risk of clinical decline during chemoradiation. Therapeutic response to chemotherapy may enable a reduction in irradiation volumes to mitigate pulmonary toxicity with concurrent taxols or motivate one to switch to an alternative regimen before or during chemoradiation. This strategy may also be considered in patients with advanced primary tumor (T4) or regional nodal burden (bulky nodes or cN2+ disease) as they are at higher risk of distant progression and theoretically may derive greater benefit from intensification of systemic therapy. The outcomes from the CROSS trial remain some of the best to date with this strategy, and similar patterns were noted in the Chinese multicenter NEOCRTEC5010 trial that demonstrated improved median survival (66 vs. 100 months), disease-free survival, and R0 resection rate with neoadjuvant vinorelbine/cisplatin with 40 Gy compared to surgery alone [102]. Future strategies seek to compare FOLFOX to paclitaxel-carboplatin with concurrent neoadjuvant radiation for both squamous and adenocarcinoma in the PROTECT-1402 trial (Preoperative Chemoradiation for Resectable Esophageal and Junctional Cancer) [103]. Given the advances in surgical and radiotherapy techniques and corresponding declines in perioperative morbidity and mortality, trimodality therapy is now a preferred approach.
3.6. Definitive Radiation and Chemoradiation
Historically, definitively treated patients generally carry significant comorbidities precluding surgery or are opposed to the morbidity of resection. Alternatively, the surgical options for patients diagnosed with cervical esophageal cancer are often limited and these patients are treated within the paradigm of head and neck cancer with definitive chemoradiation. The use of radiation alone in the modern era is generally considered to be palliative, with 5-year survival of <10% [104][105], while the importance of concurrent chemotherapy has been demonstrated historically by RTOG 8501 where, at 5 years, no survival was noted with dose escalated 64 Gy radiotherapy alone versus 27% survival with 50 Gy and concurrent four cycles of fluorouracil and cisplatin [106]. Initial forays of radiotherapy dose escalation in the definitive treatment of esophageal cancer showed disappointing clinical results. INT 0123 investigated chemoradiation with cisplatin/fluorouracil and compared 5040 cGy to dose escalation to 6480 cGy [107]. A total of 236 patients were enrolled with T1-4, N0-1 disease with 85% squamous cell carcinoma. Overall, the target volumes for this trial were relatively large, with 5-cm superior/inferior and 2-cm radial esophageal expansion as well as a 2-cm isometric expansion from tumor for the boost to gross disease. The dose escalation arm experienced a 10% treatment-related G5 toxicity rate; however, 64% of the mortality occurred prior to surpassing the dose of the control arm, potentially implicating the toxic combination of cisplatin and fluorouracil. Median survival approximated 15 months; however, 2-year locoregional failure was greater than 50%. RTOG 9207 was a phase I/II study that utilized the same control arm as INT 0123 but dose-escalated through the use of predominantly high dose rate (HDR) brachytherapy with iridium-192 to 15 Gy in three weekly fractions [108]. Unfortunately, toxicity from this strategy was high, with 12% fistula rates, 24% G4 toxicity, and 10% treatment-related deaths. Subsequently, the brachytherapy dose was dropped to 10 Gy in two weekly fractions and no fistulas were noted in the 10 patients treated with the intermediate dose. However, overall clinical outcomes remained poor, with 1-year survival of 50% and 37% local control.
With a goal of improving on the near 50% local failure rate seen in previous studies as well as overcoming high rates of persistent disease [107][108][109], dose escalation in the modern era appears to be more tolerable but still carries an elevated risk of toxicity as well as minimal evidence of clinical benefit. In preliminary results, both a sequential boost to 60 Gy with cisplatin/docetaxel [110] and simultaneous integrated boost to 61.2 Gy with carboplatin/paclitaxel [111] failed to show improvement in overall or progression free survival, despite the latter regimen carrying a 13% rate of G4 toxicity and 10% treatment-related toxicity in the high dose arm. MD Anderson has published results with their SIB technique of a high dose gross tumor volume (GTV) plus 3 mm and a planning target volume (PTV) margin of 5 mm with concurrent chemotherapy, demonstrating encouraging 66% local control at 2 years, acute G3 toxicity in 23%, and only stricture-related late G3 toxicity in 7% [112].
Newer strategies include proton therapy, which carries dosimetric benefits and higher relative biological effectiveness than photons due to the physical nature of the particle. Early retrospective data have not demonstrated an oncological benefit in comparison to photon therapy [113]; however, there is some evidence of a decrease in treatment-related toxicity which, in larger prospective studies, may carry clinical significance [114]. More work remains to further elucidate the benefits from these techniques.
3.7. Therapy for Metastatic Esophageal Cancer
Palliative radiotherapy is commonly delivered for esophageal obstruction in metastatic patients as a significant improvement in patient quality of life. Other indications are severe pain, chronic blood loss, or nausea due to tumor mass effect. While external beam radiotherapy and self-expanding metal stents (SEMS) to palliate dysphagia remain more common palliative techniques, growing literature supports the safe use of intraluminal brachytherapy for durable palliation with caution for fistulation or stenosis [115].
A meta-analysis of 53 studies (mostly RCTs) on palliation of dysphagia in inoperable esophageal cancer concluded that SEMS insertion is safe, effective, and quicker in palliating dysphagia compared to other modalities. The authors added that, “Brachytherapy might be a suitable alternative to SEMS in providing a survival advantage and possibly a better quality of life” [116].
The goal of chemotherapy in patients with stage IV metastatic esophageal cancer is to improve survival and quality of life; several chemotherapeutic agents have been tested and used in the past several decades and proven to be effective in achieving this goal. Unfortunately, survival has remained poor and rarely surpasses one year.
Combination of 5 fluorouracil (5 fu) and platinum agents is an acceptable first-line treatment option and is considered the standard of care in this setting; other regimens including paclitaxel with platinum regimen, irinotecan plus 5-fu are recommended as well. Three drug combinations such as modified DCF (Docetaxel, Cisplatin, 5 fu) are usually given to patients with high volume disease, young age, and good performance status who might benefit from a higher response rate. Single-agent treatment is recommended for patients with low volume disease and or poor performance status.
Several targeted therapies have been tested as front-line and in combination without significant improvement in overall survival until the ToGA trial was conducted. The ToGA trial was a randomized phase III trial where patients with Her-2 expressing tumors were randomized to chemotherapy with or without trastuzumab (Her-2 monoclonal antibody); patients who received the combination had better overall survival, leading to the approval of this combination in this patient population [117].
Recently, at the European Society of Medical oncology meeting in 2020 (ESMO 2020), three important trials were presented incorporating immune checkpoint inhibitors in the front-line treatment of esophageal and gastric cancer. The CheckMate 649 trial evaluated nivolumab plus chemotherapy versus chemotherapy alone as first-line treatment in patients with non-HER-2-positive advanced gastric and GE junction adenocarcinoma. The addition of Nivolumab improved overall survival and progression-free survival in patients with PD-L1 combined positive score (CPS) ≥5 tumors. Improvements were also observed in patients with PD-L1 CPS ≥1 tumors and in the overall patient population. In another study, the ATTRACTION 4 trial, which was performed only in Asian patients and the primary endpoints were designed for all-comers, rather than a specific CPS value, first-line treatment with nivolumab plus chemotherapy improved the co-primary progression-free survival endpoint, but not overall survival [118].
The third trial, presented at the same meeting, the KEYNOTE 590 trial, examined first-line chemotherapy with or without pembrolizumab in patients with squamous cell carcinoma of the esophagus, adenocarcinoma of the esophagus, or Siewert-type GE junction adenocarcinoma. It demonstrated that pembrolizumab plus chemotherapy improved overall survival in patients with squamous cell carcinoma of the esophagus with PD-L1 CPS ≥10 tumors, all squamous cell carcinomas, all patients with CPS ≥10, and the study population as a whole. Progression-free survival was also improved. It is expected that these trials will change the landscape of the treatment of esophageal cancer worldwide [118].
Several chemotherapy and targeted agents have been studied in the second-line treatment of esophageal and gastric cancer and beyond. Chemotherapy improves survival compared to placebo, single-agent paclitaxel with or without ramucirumab, a monoclonal antibody targeting the VEGF pathway, which is a commonly used second-line regimen with overall survival approaching 9 months. Single-agent ramucirumab, irinotecan, and immune checkpoint inhibitors are all approved for the second-line treatment as well, with modest benefits. Patients with microsatellite instable disease should be treated with immune check point inhibitors at any point during the course of their disease [118].
Several ongoing trials are testing other targeted therapies and are beyond the scope of this review.
Patients with inoperable esophageal cancer and with high-grade dysphagia were randomized to receive a self-expandable metal stent (SEMS) alone (Group I), versus a combination of SEMS followed by external beam radiation (over 2 weeks) (Group II). Dysphagia scores improved significantly in both groups following stent insertion. However, dysphagia relief was more sustained in Group II than in Group I (7 vs. 3 months, p = 0.002), and overall median survival was significantly higher in Group II than in Group I [119]. A recent meta-analysis of several RCTs evaluating the efficacy of SEMS alone vs. SEMS combined with radiotherapy concluded that the combination of SEMS and radiation significantly improves the overall survival as well as leading to improvements in quality of life scores [120].
In summary, the current trend in the literature shows that the best oncological outcomes are associated with trimodality therapy with neoadjuvant chemoradiation. This has been supported by recent meta-analyses that conclude that compared with neoadjuvant chemotherapy, neoadjuvant chemoradiotherapy should be recommended, with a significant long-term survival benefit in patients with cancer of the esophagus or the GEJ [121][122]. While treatments and outcomes are improving, a large proportion of patients fail locally, and addition of biologically targeted agents or local therapy intensification may prove to be beneficial.
For patients who are considered to be surgical candidates, neoadjuvant chemotherapy with weekly carboplatin and paclitaxel in combination with radiation followed by surgical resection is the standard of care. This treatment is based on the CROSS trial, where neoadjuvant therapy resulted in improved overall survival compared to esophagectomy alone [97]. Definitive chemotherapy and radiation is a reasonable option for patients who are not surgical candidates or with cervical/mid-esophageal SCC. On rare occasions, patients who are thought to have T1N0 disease are upstaged during esophagectomy; the role of adjuvant therapy in this setting is not clear and carries significant toxicity. The addition of Her-2 targeted therapy with trastuzumab (Her-2 antibody) to the neoadjuvant therapy did not result in improved outcome [123]. Several ongoing trials are currently evaluating the addition of immune checkpoint inhibitors in both the neoadjuvant and the adjuvant settings. These results are highly anticipated.
As previously described, neoadjuvant radiation therapy in addition to chemotherapy plays an important role in the multimodality treatment of locally advanced esophageal cancer. Fiducial markers have been integrated into the management of multiple malignancies to guide more precise delivery of radiation therapy (RT).
This entry is adapted from the peer-reviewed paper 10.3390/cancers13030582
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424.
- Van Cutsem, E.; Sagaert, X.; Topal, B.; Haustermans, K.; Prenen, H. Gastric cancer. Lancet 2016, 388, 2654–2664.
- Arnold, M.; Park, J.Y.; Camargo, M.C.; Lunet, N.; Forman, D.; Soerjomataram, I. Is gastric cancer becoming a rare disease? A global assessment of predicted incidence trends to 2035. Gut 2020, 69, 823–829.
- Laurén, P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. Acta Pathol. Microbiol. Scand. 1965, 64, 31–49.
- Adam, B.; Pech, O.; Steckstor, M.; Tannapfel, A.; Riphaus, A. Gastric Mucosa-Associated Lymphoid Tissue Lymphoma. Video J. Encycl. GI Endosc. 2013, 1, 174–175.
- Sanon, M.; Taylor, D.C.; Coombs, J.D.; Sirulnik, L.; Rubin, J.L.; Bollu, V. Epidemiology, survival, and costs of localized gastrointestinal stromal tumors. Int. J. Gen. Med. 2011, 4, 121–130.
- Que, J.; Garman, K.S.; Souza, R.F.; Spechler, S.J. Pathogenesis and Cells of Origin of Barrett’s Esophagus. Gastroenterology 2019, 157, 349–364.e1.
- Shaheen, N.J.; E Richter, J. Barrett’s oesophagus. Lancet 2009, 373, 850–861.
- Correa, P.; Piazuelo, M.B.; Wilson, K.T. Pathology of Gastric Intestinal Metaplasia: Clinical Implications. Am. J. Gastroenterol. 2010, 105, 493–498.
- Leung, W.K. Risk Factors Associated with the Development of Intestinal Metaplasia in First-Degree Relatives of Gastric Cancer Patients. Cancer Epidemiol. Biomark. Prev. 2005, 14, 2982–2986.
- Uemura, N.; Okamoto, S.; Yamamoto, S.; Matsumura, N.; Yamaguchi, S.; Yamakido, M.; Taniyama, K.; Sasaki, N.; Schlemper, R.J. Helicobacter pyloriInfection and the Development of Gastric Cancer. N. Engl. J. Med. 2001, 345, 784–789.
- Wang, Z.; Kambhampati, S.; Cheng, Y.; Ma, K.; Simsek, C.; Tieu, A.H.; Abraham, J.M.; Liu, X.; Prasath, V.; Duncan, M.; et al. Methylation Biomarker Panel Performance in EsophaCap Cytology Samples for Diagnosing Barrett’s Esophagus: A Prospective Validation Study. Clin. Cancer Res. 2019, 25, 2127–2135.
- Fitzgerald, R.C.; Di Pietro, M.; O’Donovan, M.; Maroni, R.; Muldrew, B.; Debiram-Beecham, I.; Gehrung, M.; Offman, J.; Tripathi, M.; Smith, S.G.; et al. Cytosponge-trefoil factor 3 versus usual care to identify Barrett’s oesophagus in a primary care setting: A multicentre, pragmatic, randomised controlled trial. Lancet 2020, 396, 333–344.
- Yamamoto, H.; Watanabe, Y.; Sato, Y.; Maehata, T.; Itoh, F. Non-Invasive Early Molecular Detection of Gastric Cancers. Cancers 2020, 12, 2880.
- Desai, M.; Saligram, S.; Gupta, N.; Vennalaganti, P.; Bansal, A.; Choudhary, A.; Vennelaganti, S.; He, J.; Titi, M.; Maselli, R.; et al. Efficacy and safety outcomes of multimodal endoscopic eradication therapy in Barrett’s esophagus-related neoplasia: A systematic review and pooled analysis. Gastrointest. Endosc. 2017, 85, 482–495.e4.
- Waddingham, W.; Nieuwenburg, S.A.V.; Carlson, S.; Rodriguez-Justo, M.; Spaander, M.; Kuipers, E.J.; Jansen, M.; Graham, D.G.; Banks, M. Recent advances in the detection and management of early gastric cancer and its precursors. Front. Gastroenterol. 2020.
- Pimentel-Nunes, P.; Dinis-Ribeiro, M.; Soares, J.B.; Marcos-Pinto, R.; Santos, C.; Rolanda, C.; Bastos, R.P.; Areia, M.; Afonso, L.; Bergman, J.; et al. A multicenter validation of an endoscopic classification with narrow band imaging for gastric precancerous and cancerous lesions. Endoscopy 2012, 44, 236–246.
- Sharma, P.; Hawes, R.H.; Bansal, A.; Gupta, N.; Curvers, W.; Rastogi, A.; Singh, M.; Hall, M.; Mathur, S.C.; Wani, S.; et al. Standard endoscopy with random biopsies versus narrow band imaging targeted biopsies in Barrett’s oesophagus: a prospective, international, randomised controlled trial. Gut 2012, 62, 15–21.
- Ajani, J.A.; D’Amico, T.A.; Bentrem, D.J.; Chao, J.; Corvera, C.; Das, P.; Denlinger, C.S.; Enzinger, P.C.; Fanta, P.; Farjah, F.; et al. Esophageal and Esophagogastric Junction Cancers, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2019, 17, 855–883.
- Song, B.G.; Min, Y.W.; Cha, R.R.; Lee, H.; Min, B.-H.; Lee, J.H.; Rhee, P.-L.; Kim, J.J. Endoscopic submucosal dissection under general anesthesia for superficial esophageal squamous cell carcinoma is associated with better clinical outcomes. BMC Gastroenterol. 2018, 18, 80.
- Van Hagen, P.; Spaander, M.C.W.; Van Der Gaast, A.; Van Rij, C.M.; Tilanus, H.W.; Van Lanschot, J.J.B.; Wijnhoven, B.P.L. Impact of a multidisciplinary tumour board meeting for upper-GI malignancies on clinical decision making: A prospective cohort study. Int. J. Clin. Oncol. 2011, 18, 214–219.
- Basta, Y.L.; Bolle, S.; Fockens, P.; Tytgat, K.M.A.J. The Value of Multidisciplinary Team Meetings for Patients with Gastrointestinal Malignancies: A Systematic Review. Ann. Surg. Oncol. 2017, 24, 2669–2678.
- Subasinghe, D.; Acott, N.; Kumarasinghe, M.P. A survival guide to HER2 testing in gastric/gastroesophageal junction carcinoma. Gastrointest. Endosc. 2019, 90, 44–54.
- Turner, E.S.; Turner, J.R. Expanding the Lauren Classification: A New Gastric Cancer Subtype? Gastroenterology 2013, 145, 505–508.
- Watanabe, M. Risk factors and molecular mechanisms of esophageal cancer: differences between the histologic subtype. J. Cancer Metastasis Treat. 2015.
- Abnet, C.C.; Arnold, M.; Wei, W. Epidemiology of Esophageal Squamous Cell Carcinoma. Gastroenterology 2018, 154, 360–373.
- Arnold, M.; Soerjomataram, I.; Ferlay, J.; Forman, D. Global incidence of oesophageal cancer by histological subtype in 2012. Gut 2015, 64, 381–387.
- Cook, M.B.; Chow, W.-H.; Devesa, S.S. Oesophageal cancer incidence in the United States by race, sex, and histologic type, 1977–2005. Br. J. Cancer 2009, 101, 855–859.
- Coleman, H.G.; Xie, S.-H.; Lagergren, J. The Epidemiology of Esophageal Adenocarcinoma. Gastroenterology 2018, 154, 390–405.
- Eluri, S.; Shaheen, N.J. Barrett’s esophagus: Diagnosis and management. Gastrointest. Endosc. 2017, 85, 889–903.
- Ireland, C.J.; Thompson, S.K.; Laws, T.A.; Esterman, A. Risk factors for Barrett’s esophagus: A scoping review. Cancer Causes Control. 2016, 27, 301–323.
- Sharma, P.; Shaheen, N.J.; Katzka, D.; Bergman, J.J. AGA Clinical Practice Update on Endoscopic Treatment of Barrett’s Esophagus With Dysplasia and/or Early Cancer: Expert Review. Gastroenterology 2020, 158, 760–769.
- Bhat, S.; Coleman, H.G.; Yousef, F.; Johnston, B.T.; McManus, D.T.; Gavin, A.T.; Murray, L.J. Risk of Malignant Progression in Barrett’s Esophagus Patients: Results from a Large Population-Based Study. J. Natl. Cancer Inst. 2011, 103, 1049–1057.
- Shaheen, N.J.; Sharma, P.; Overholt, B.F.; Wolfsen, H.C.; Sampliner, R.E.; Wang, K.K.; Galanko, J.A.; Bronner, M.P.; Goldblum, J.R.; Bennett, A.E.; et al. Radiofrequency Ablation in Barrett’s Esophagus with Dysplasia. N. Engl. J. Med. 2009, 360, 2277–2288.
- Shaheen, N.J.; Falk, G.W.; Iyer, P.G.; Gerson, L.B. ACG Clinical Guideline: Diagnosis and Management of Barrett’s Esophagus. Am. J. Gastroenterol. 2016, 111, 30–50.
- Furneri, G.; Klausnitzer, R.; Haycock, L.; Ihara, Z. Economic value of narrow-band imaging versus white light endoscopy for the diagnosis and surveillance of Barrett’s esophagus: Cost-consequence model. PLOS ONE 2019, 14, e0212916.
- Xiong, Y.-Q.; Ma, S.-J.; Hu, H.-Y.; Ge, J.; Zhou, L.-Z.; Huo, S.-T.; Qiu, M.; Chen, Q. Comparison of narrow-band imaging and confocal laser endomicroscopy for the detection of neoplasia in Barrett’s esophagus: A meta-analysis. Clin. Res. Hepatol. Gastroenterol. 2018, 42, 31–39.
- Puli, S.R.; Reddy, J.B.; Bechtold, M.L.; Antillon, D.; A Ibdah, J.; Antillon, M.R. Staging accuracy of esophageal cancer by endoscopic ultrasound: A meta-analysis and systematic review. World J. Gastroenterol. 2008, 14, 1479–1490.
- Qumseya, B.J.; Wolfsen, H.C. The Role of Endoscopic Ultrasound in the Management of Patients with Barrett’s Esophagus and Superficial Neoplasia. Gastrointest. Endosc. Clin. North Am. 2017, 27, 471–480.
- Thosani, N.; Singh, H.; Kapadia, A.; Ochi, N.; Lee, J.H.; Ajani, J.; Swisher, S.G.; Hofstetter, W.L.; Guha, S.; Bhutani, M.S. Diagnostic accuracy of EUS in differentiating mucosal versus submucosal invasion of superficial esophageal cancers: a systematic review and meta-analysis. Gastrointest. Endosc. 2012, 75, 242–253.
- Luo, L.-N.; He, L.-J.; Gao, X.-Y.; Huang, X.-X.; Shan, H.-B.; Luo, G.-Y.; Li, Y.; Lin, S.-Y.; Wang, G.-B.; Zhang, R.; et al. Endoscopic Ultrasound for Preoperative Esophageal Squamous Cell Carcinoma: A Meta-Analysis. PLOS ONE 2016, 11, e0158373.
- Flamen, P.; Lerut, A.; Van Cutsem, E.; De Wever, W.; Peeters, M.; Stroobants, S.; Dupont, P.; Bormans, G.; Hiele, M.; De Leyn, P.; et al. Utility of Positron Emission Tomography for the Staging of Patients With Potentially Operable Esophageal Carcinoma. J. Clin. Oncol. 2000, 18, 3202–3210.
- Goodman, K.A.; Niedzwiecki, D.; Hall, N.; Bekaii-Saab, T.S.; Ye, X.; Meyers, M.O.; Mitchell-Richards, K.; Boffa, D.J.; Frankel, W.L.; Venook, A.P.; et al. Initial results of CALGB 80803 (Alliance): A randomized phase II trial of PET scan-directed combined modality therapy for esophageal cancer. J. Clin. Oncol. 2017, 35, 1.
- Johnston, M.H.; Eastone, J.A.; Horwhat, J.; Cartledge, J.; Mathews, J.S.; Foggy, J.R. Cryoablation of Barrett’s esophagus: A pilot study. Gastrointest. Endosc. 2005, 62, 842–848.
- Phoa, K.N.; Van Vilsteren, F.G.I.; Weusten, B.L.; Bisschops, R.; Schoon, E.J.; Ragunath, K.; Fullarton, G.; Di Pietro, M.; Ravi, N.; Visser, M.; et al. Radiofrequency Ablation vs Endoscopic Surveillance for Patients With Barrett Esophagus and Low-Grade Dysplasia. JAMA 2014, 311, 1209–1217.
- Cotton, C.C.; Wolf, W.A.; Overholt, B.F.; Li, N.; Lightdale, C.J.; Wolfsen, H.C.; Pasricha, S.; Wang, K.K.; Shaheen, N.J.; Sampliner, R.E.; et al. Late Recurrence of Barrett’s Esophagus After Complete Eradication of Intestinal Metaplasia is Rare: Final Report From Ablation in Intestinal Metaplasia Containing Dysplasia Trial. Gastroenterology 2017, 153, 681–688.e2.
- Manner, H.; Pech, O.; Heldmann, Y.; May, A.; Pohl, J.; Behrens, A.; Gossner, L.; Stolte, M.; Vieth, M.; Ell, C. Efficacy, Safety, and Long-term Results of Endoscopic Treatment for Early Stage Adenocarcinoma of the Esophagus With Low-risk sm1 Invasion. Clin. Gastroenterol. Hepatol. 2013, 11, 630–635.
- Conio, M.; Repici, A.; Cestari, R.; Blanchi, S.; Lapertosa, G.; Missale, G.; Della Casa, D.; Villanacci, V.; Calandri, P.G.; Filiberti, R. Endoscopic mucosal resection for high-grade dysplasia and intramucosal carcinoma in Barrett’s esophagus: An Italian experience. World J. Gastroenterol. 2005, 11, 6650–6655.
- Bourke, M.J. Mucosal resection in the upper gastrointestinal tract. Tech. Gastrointest. Endosc. 2010, 12, 18–25.
- Moss, A.; Bourke, M.J.; Hourigan, L.F.; Gupta, S.; Swan, M.P.; Hopper, A.D.; Kwan, V.; Bailey, A.; Williams, S.J. Endoscopic Mucosal Resection (EMR) for Barrett’s High Grade Dysplasia (HGD) and Early Esophageal Adenocarcinoma (EAC): An Essential Staging Procedure with Long-Term Therapeutic Benefit. Gastrointest. Endosc. 2009, 69, AB348.
- Jin, X.-F.; Chai, T.-H.; Gai, W.; Chen, Z.-S.; Guo, J.-Q. Multiband Mucosectomy Versus Endoscopic Submucosal Dissection for Treatment of Squamous Intraepithelial Neoplasia of the Esophagus. Clin. Gastroenterol. Hepatol. 2016, 14, 948–955.
- Rajaram, R.; Hofstetter, W.L. Mucosal Ablation Techniques for Barrett’s Esophagus and Early Esophageal Cancer. Thorac. Surg. Clin. 2018, 28, 473–480.
- Inoue, H.; Endo, M.; Takeshita, K.; Yoshino, K.; Muraoka, Y.; Yoneshima, H. A new simplified technique of endoscopic esophageal mucosal resection using a cap-fitted panendoscope (EMRC). Surg. Endosc. 1992, 6, 264–265.
- Espinel, J.; Pinedo, E.; Ojeda, V.; Del Rio, M.G. Multiband mucosectomy for advanced dysplastic lesions in the upper digestive tract. World J. Gastrointest. Endosc. 2015, 7, 370–380.
- Wani, S.; Qumseya, B.; Sultan, S.; Agrawal, D.; Chandrasekhara, V.; Harnke, B.; Kothari, S.; McCarter, M.; Shaukat, A.; Wang, A.; et al. Endoscopic eradication therapy for patients with Barrett’s esophagus–associated dysplasia and intramucosal cancer. Gastrointest. Endosc. 2018, 87, 907–931.e9.
- Ahmed, Y.; Othman, M. EMR/ESD: Techniques, Complications, and Evidence. Curr. Gastroenterol. Rep. 2020, 22, 1–12.
- Davison, J.M.; Landau, M.S.; Luketich, J.D.; McGrath, K.M.; Foxwell, T.J.; Landsittel, D.P.; Gibson, M.K.; Nason, K.S. A Model Based on Pathologic Features of Superficial Esophageal Adenocarcinoma Complements Clinical Node Staging in Determining Risk of Metastasis to Lymph Nodes. Clin. Gastroenterol. Hepatol. 2016, 14, 369–377.e3.
- Othman, M.O.; Lee, J.H.; Wang, K. AGA Clinical Practice Update on the Utility of Endoscopic Submucosal Dissection in T1b Esophageal Cancer: Expert Review. Clin. Gastroenterol. Hepatol. 2019, 17, 2161–2166.
- Zhang, Y.; Ding, H.; Chen, T.; Zhang, X.; Chen, W.-F.; Li, Q.; Yao, L.; Korrapati, P.; Jin, X.-J.; Zhang, Y.-X.; et al. Outcomes of Endoscopic Submucosal Dissection vs Esophagectomy for T1 Esophageal Squamous Cell Carcinoma in a Real-World Cohort. Clin. Gastroenterol. Hepatol. 2019, 17, 73–81.e3.
- Aadam, A.A.; Abe, S. Endoscopic submucosal dissection for superficial esophageal cancer. Dis. Esophagus 2018, 31.
- Sgourakis, G.; Gockel, I.; Lang, H. Endoscopic and surgical resection of T1a/T1b esophageal neoplasms: A systematic review. World J. Gastroenterol. 2013, 19, 1424–1437.
- Komeda, Y.; Bruno, M.; Koch, A. EMR is not inferior to ESD for early Barrett’s and EGJ neoplasia: An extensive review on outcome, recurrence and complication rates. Endosc. Int. Open 2014, 2, E58–E64.
- Kaya, D.M.; Harada, K.; Das, P.; Weston, B.; Sagebiel, T.; Thomas, I.; Wang, X.; Murphy, M.A.B.; Minsky, B.D.; Estrella, J.S.; et al. 101 Long-Term Survivors Who Had Metastatic Gastroesophageal Cancer and Received Local Consolidative Therapy. Oncology 2017, 93, 243–248.
- Berry, M.F. Esophageal cancer: Staging system and guidelines for staging and treatment. J. Thorac. Dis. 2014, 6, S289–S297.
- van Boxel, G.I.; Kingma, B.F.; Voskens, F.J.; Ruurda, J.P.; van Hillegersberg, R. Robotic-assisted minimally invasive esoph-agectomy: Past, present and future. J. Thorac. Dis. 2020, 12, 54–62.
- Van Der Sluis, P.C.; Schizas, D.; Liakakos, T.; Van Hillegersberg, R. Minimally Invasive Esophagectomy. Dig. Surg. 2020, 37, 93–100.
- Tepper, J.; Krasna, M.J.; Niedzwiecki, D.; Hollis, D.; Reed, C.E.; Goldberg, R.; Kiel, K.; Willett, C.; Sugarbaker, D.; Mayer, R. Phase III Trial of Trimodality Therapy With Cisplatin, Fluorouracil, Radiotherapy, and Surgery Compared With Surgery Alone for Esophageal Cancer: CALGB 9781. J. Clin. Oncol. 2008, 26, 1086–1092.
- Ohtsu, A. Chemoradiotherapy for esophageal cancer: current status and perspectives. Int. J. Clin. Oncol. 2004, 9, 444–450.
- Jethwa, K.R.; Haddock, M.G.; Tryggestad, E.J.; Hallemeier, C.L. The emerging role of proton therapy for esophagus cancer. J. Gastrointest. Oncol. 2020, 11, 144–156.
- Lindenmann, J.; Matzi, V.; Neuboeck, N.; Anegg, U.; Baumgartner, E.; Maier, A.; Smolle, J.; Smolle-Juettner, F.M. Individualized, multimodal palliative treatment of inoperable esophageal cancer: Clinical impact of photodynamic therapy resulting in prolonged survival. Lasers Surg. Med. 2012, 44, 189–198.
- Lloyd, S.; Chang, B. Current strategies in chemoradiation for esophageal cancer. J. Gastrointest. Oncol. 2014, 5, 156–165.
- Xi, M.; Lin, S.H. Recent advances in intensity modulated radiotherapy and proton therapy for esophageal cancer. Expert Rev. Anticancer. Ther. 2017, 17, 635–646.
- Kole, T.P.; Aghayere, O.; Kwah, J.; Yorke, E.D.; Goodman, K.A. Comparison of Heart and Coronary Artery Doses Associated With Intensity-Modulated Radiotherapy Versus Three-Dimensional Conformal Radiotherapy for Distal Esophageal Cancer. Int. J. Radiat. Oncol. 2012, 83, 1580–1586.
- Nutting, C.M.; Bedford, J.L.; Cosgrove, V.P.; Tait, D.M.; Dearnaley, D.; Webb, S. A comparison of conformal and intensity-modulated techniques for oesophageal radiotherapy. Radiother. Oncol. 2001, 61, 157–163.
- Tonison, J.J.; Fischer, S.G.; Viehrig, M.; Welz, S.; Boeke, S.; Zwirner, K.; Klumpp, B.; Braun, L.H.; Zips, D.; Gani, C. Radiation Pneumonitis after Intensity-Modulated Radiotherapy for Esophageal Cancer: Institutional Data and a Systematic Review. Sci. Rep. 2019, 9, 2255.
- Pao, T.-H.; Chang, W.-L.; Chiang, N.-J.; Chang, J.S.-M.; Lin, C.-Y.; Lai, W.-W.; Tseng, Y.-L.; Yen, Y.-T.; Chung, T.-J.; Lin, F.-C. Cardiac radiation dose predicts survival in esophageal squamous cell carcinoma treated by definitive concurrent chemotherapy and intensity modulated radiotherapy. Radiat. Oncol. 2020, 15, 1–10.
- Ogino, I.; Watanabe, S.; Iwahashi, N.; Kosuge, M.; Sakamaki, K.; Kunisaki, C.; Kimura, K. Symptomatic radiation-induced cardiac disease in long-term survivors of esophageal cancer. Strahlenther. Onkol. 2016, 192, 359–367.
- Wang, X.; Palaskas, N.L.; Yusuf, S.W.; Abe, J.-I.; Lopez-Mattei, J.; Banchs, J.; Gladish, G.W.; Lee, P.; Liao, Z.; Deswal, A.; et al. Incidence and Onset of Severe Cardiac Events After Radiotherapy for Esophageal Cancer. J. Thorac. Oncol. 2020, 15, 1682–1690.
- Dhadham, G.C.; Hoffe, S.; Harris, C.L.; Klapman, J. Endoscopic ultrasound-guided fiducial marker placement for image-guided radiation therapy without fluoroscopy: Safety and technical feasibility. Endosc. Int. Open 2016, 4, E378–E382.
- DiMaio, C.J.; Nagula, S.; Goodman, K.A.; Ho, A.Y.; Markowitz, A.J.; Schattner, M.A.; Gerdes, H. EUS-guided fiducial placement for image-guided radiation therapy in GI malignancies by using a 22-gauge needle (with). Gastrointest. Endosc. 2010, 71, 1204–1210.
- Fernandez, D.C.; Hoffe, S.; Barthel, J.S.; Vignesh, S.; Klapman, J.B.; Harris, C.; Almhanna, K.; Biagioli, M.C.; Meredith, K.; Feygelman, V.; et al. Stability of endoscopic ultrasound-guided fiducial marker placement for esophageal cancer target delineation and image-guided radiation therapy. Pr. Radiat. Oncol. 2013, 3, 32–39.
- Oliver, J.A.; Venkat, P.; Frakes, J.M.; Klapman, J.; Harris, C.; Montilla-Soler, J.; Dhadham, G.C.; Altazi, B.A.; Zhang, G.G.; Moros, E.G.; et al. Fiducial markers coupled with 3D PET/CT offer more accurate radiation treatment delivery for locally advanced esophageal cancer. Endosc. Int. Open 2017, 5, E496–E504.
- Machiels, M.; Van Hooft, J.; Jin, P.; Henegouwen, M.I.V.B.; Van Laarhoven, H.M.; Alderliesten, T.; Hulshof, M.C. Endoscopy/EUS-guided fiducial marker placement in patients with esophageal cancer: A comparative analysis of 3 types of markers. Gastrointest. Endosc. 2015, 82, 641–649.
- Wang, J.; Lin, S.H.; Dong, L.; Balter, P.; Mohan, R.; Komaki, R.; Cox, J.D.; Starkschall, G. Quantifying the Interfractional Displacement of the Gastroesophageal Junction During Radiation Therapy for Esophageal Cancer. Int. J. Radiat. Oncol. 2012, 83, e273–e280.
- Wu, A.J.; Bosch, W.R.; Chang, D.T.; Hong, T.S.; Jabbour, S.K.; Kleinberg, L.R.; Mamon, H.J.; Thomas, C.R.; Goodman, K.A. Expert Consensus Contouring Guidelines for Intensity Modulated Radiation Therapy in Esophageal and Gastroesophageal Junction Cancer. Int. J. Radiat. Oncol. 2015, 92, 911–920.
- Launois, B.; Delarue, D.; Campion, J.P.; Kerbaol, M. Preoperative radiotherapy for carcinoma of the esophagus. Surgery, Gynecol. Obstet. 1981, 153, 690–692.
- Wang, M.; Gu, X.Z.; Yin, W.B.; Huang, G.J.; Wang, L.J.; Zhang, D.W. Randomized clinical trial on the combination of preoper-ative irradiation and surgery in the treatment of esophageal carcinoma: Report on 206 patients. Int. J. Radiat. Oncol. Biol. Phys. 1989, 16, 325–327.
- Gignoux, M.; Roussel, A.; Paillot, B.; Gillet, M.; Schlag, P.; Favre, J.-P.; Dalesio, O.; Buyse, M.; Duez, N. The value of preoperative radiotherapy in esophageal cancer: Results of a study of the E.O.R.T.C. World J. Surg. 1987, 11, 426–432.
- Nygaard, K.; Hagen, S.; Hansen, H.S.; Hatlevoll, R.; Hultborn, R.; Jakobsen, A.; Mäntyla, M.; Modig, H.; Munck-Wikland, E.; Rosengren, B.; et al. Pre-operative radiotherapy prolongs survival in operable esophageal carcinoma: A randomized, multicenter study of pre-operative radiotherapy and chemotherapy. The second scandinavian trial in esophageal cancer. World J. Surg. 1992, 16, 1104–1109.
- Arnott, S.J.; Duncan, W.; Gignoux, M.; Girling, D.J.; Hansen, H.S.; Launois, B.; Nygaard, K.; Parmar, M.K.; Roussel, A.; Spiliopoulos, G.; et al. Preoperative radiotherapy in esophageal carcinoma: A meta-analysis using individual patient data (oesophageal cancer collaborative group). Int. J. Radiat. Oncol. 1998, 41, 579–583.
- Batra, T.K.; Pai, E.; Singh, R.; Francis, N.J.; Pandey, M. Neoadjuvant strategies in resectable carcinoma esophagus: A meta-analysis of randomized trials. World J. Surg. Oncol. 2020, 18, 1–10.
- Fok, M.; Sham, J.S.; Choy, D.; Cheng, S.W.; Wong, J. Postoperative radiotherapy for carcinoma of the esophagus: a prospective, randomized controlled study. Surgery 1993, 113, 138–147.
- Ténière, P.; Hay, J.M.; Fingerhut, A.; Fagniez, P.L. Postoperative radiation therapy does not increase survival after curative resection for squamous cell carcinoma of the middle and lower esophagus as shown by a multicenter controlled trial. French University Association for Surgical Research. Surgery, Gynecol. Obstet. 1991, 173, 123–130.
- Hsu, P.-K.; Huang, C.-S.; Wang, B.-Y.; Wu, Y.-C.; Hsu, W.-H. Survival Benefits of Postoperative Chemoradiation for Lymph Node–Positive Esophageal Squamous Cell Carcinoma. Ann. Thorac. Surg. 2014, 97, 1734–1741.
- Rucker, A.J.; Raman, V.; Jawitz, O.K.; Voigt, S.L.; Harpole, D.H.; D’Amico, T.A.; Tong, B.C. The Impact of Adjuvant Therapy on Survival After Esophagectomy for Node-negative Esophageal Adenocarcinoma. Ann. Surg. 2020.
- Ni, W.; Yu, S.; Zhang, W.; Xiao, Z.; Zhou, Z.; Chen, D.; Feng, Q.; Liang, J.; Lv, J.; Gao, S.; et al. A phase-II/III randomized controlled trial of adjuvant radiotherapy or concurrent chemoradiotherapy after surgery versus surgery alone in patients with stage-IIB/III esophageal squamous cell carcinoma. BMC Cancer 2020, 20, 1–8.
- Shapiro, J.; Van Lanschot, J.J.B.; Hulshof, M.C.C.M.; Van Hagen, P.; Henegouwen, M.I.V.B.; Wijnhoven, B.P.L.; Van Laarhoven, H.W.M.; Nieuwenhuijzen, G.A.P.; Hospers, G.A.P.; Bonenkamp, J.J.; et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): Long-term results of a randomised controlled trial. Lancet Oncol. 2015, 16, 1090–1098.
- Stahl, M.; Walz, M.K.; Riera-Knorrenschild, J.; Stuschke, M.; Sandermann, A.; Bitzer, M.; Wilke, H.; Budach, W. Preoperative chemotherapy versus chemoradiotherapy in locally advanced adenocarcinomas of the oesophagogastric junction (POET): Long-term results of a controlled randomised trial. Eur. J. Cancer 2017, 81, 183–190.
- Klevebro, F.; Von Döbeln, G.A.; Wang, N.; Johnsen, G.; Jacobsen, A.-B.; Friesland, S.; Hatlevoll, I.; Glenjen, N.I.; Lind, P.; Tsai, J.A.; et al. A randomized clinical trial of neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy for cancer of the oesophagus or gastro-oesophageal junction. Ann. Oncol. 2016, 27, 660–667.
- Oppedijk, V.; Van Der Gaast, A.; Van Lanschot, J.J.B.; Van Hagen, P.; Van Os, R.; Van Rij, C.M.; Van Der Sangen, M.J.; Beukema, J.C.; Rütten, H.; Spruit, P.H.; et al. Patterns of Recurrence After Surgery Alone Versus Preoperative Chemoradiotherapy and Surgery in the CROSS Trials. J. Clin. Oncol. 2014, 32, 385–391.
- Noordman, B.J.; Verdam, M.G.E.; Lagarde, S.M.; Hulshof, M.C.C.M.; Van Hagen, P.; Henegouwen, M.I.V.B.; Wijnhoven, B.P.L.; Van Laarhoven, H.W.M.; Nieuwenhuijzen, G.A.P.; Hospers, G.A.; et al. Effect of Neoadjuvant Chemoradiotherapy on Health-Related Quality of Life in Esophageal or Junctional Cancer: Results From the Randomized CROSS Trial. J. Clin. Oncol. 2018, 36, 268–275.
- Yang, H.; Liu, H.; Chen, Y.; Zhu, C.; Fang, W.; Yu, Z.; Mao, W.; Xiang, J.; Han, Y.; Chen, Z.; et al. Neoadjuvant Chemoradiotherapy Followed by Surgery Versus Surgery Alone for Locally Advanced Squamous Cell Carcinoma of the Esophagus (NEOCRTEC5010): A Phase III Multicenter, Randomized, Open-Label Clinical Trial. J. Clin. Oncol. 2018, 36, 2796–2803.
- Messager, M.; Mirabel, X.; Tresch, E.; Paumier, A.; Vendrely, V.; Dahan, L.; Glehen, O.; Vasseur, F.; Lacornerie, T.; Piessen, G.; et al. Preoperative chemoradiation with paclitaxel-carboplatin or with fluorouracil-oxaliplatin-folinic acid (FOLFOX) for resectable esophageal and junctional cancer: the PROTECT-1402, randomized phase 2 trial. BMC Cancer 2016, 16, 318.
- Okawa, T.; Kita, M.; Tanaka, M.; Ikeda, M. Results of radiotherapy for inoperable locally advanced esophageal cancer. Int. J. Radiat. Oncol. 1989, 17, 49–54.
- De-Ren, S.; Sun, D.R. Ten-year follow-up of esophageal cancer treated by radical radiation therapy: Analysis of 869 patients. Int. J. Radiat. Oncol. 1989, 16, 329–334.
- Herskovic, A.; Martz, K.; Al-Sarraf, M.; Leichman, L.; Brindle, J.; Vaitkevicius, V.; Cooper, J.; Byhardt, R.; Davis, L.; Emami, B. Combined Chemotherapy and Radiotherapy Compared with Radiotherapy Alone in Patients with Cancer of the Esophagus. N. Engl. J. Med. 1992, 326, 1593–1598.
- Minsky, B.D.; Pajak, T.F.; Ginsberg, R.J.; Pisansky, T.M.; Martenson, J.; Komaki, R.; Okawara, G.; Rosenthal, S.A.; Kelsen, D.P. INT 0123 (Radiation Therapy Oncology Group 94-05) Phase III Trial of Combined-Modality Therapy for Esophageal Cancer: High-Dose Versus Standard-Dose Radiation Therapy. J. Clin. Oncol. 2002, 20, 1167–1174.
- E Gaspar, L.; Winter, K.; I Kocha, W.; Coia, L.R.; Herskovic, A.; Graham, M. A phase I/II study of external beam radiation, brachytherapy, and concurrent chemotherapy for patients with localized carcinoma of the esophagus (Radiation Therapy Oncology Group Study 9207): Final report. Cancer 2000, 88, 988–995.
- Cooper, J.S.; Guo, M.D.; Herskovic, A.; Macdonald, J.S.; Martenson, J.J.A.; Al-Sarraf, M.; Byhardt, R.; Russell, A.H.; Beitler, J.J.; Spencer, S.; et al. Chemoradiotherapy of Locally Advanced Esophageal Cancer. JAMA 1999, 281, 1623–1627.
- Xu, Y.; Zhu, W.; Zheng, X.; Wang, W.; Li, J.; Huang, R.; He, H.; Chen, J.; Liu, L.; Sun, Z.; et al. A multi-center, randomized, prospective study evaluating the optimal radiation dose of definitive concurrent chemoradiation for inoperable esophageal squamous cell carcinoma. J. Clin. Oncol. 2018, 36, 4013.
- Hulshof, M.C.; Geijsen, D.; Rozema, T.; Oppedijk, V.; Buijsen, J.; Neelis, K.J.; Nuyttens, J.; Van Der Sangen, M.; Jeene, P.; Reinders, J.; et al. A randomized controlled phase III multicenter study on dose escalation in definitive chemoradiation for patients with locally advanced esophageal cancer: ARTDECO study. J. Clin. Oncol. 2020, 38, 281.
- Chen, D.; Menon, H.; Verma, V.; Seyedin, S.N.; Ajani, J.A.; Hofstetter, W.L.; Nguyen, Q.-N.; Chang, J.Y.; Gomez, D.R.; Amini, A.; et al. Results of a Phase 1/2 Trial of Chemoradiotherapy With Simultaneous Integrated Boost of Radiotherapy Dose in Unresectable Locally Advanced Esophageal Cancer. JAMA Oncol. 2019, 5, 1597–1604.
- Bhangoo, R.S.; DeWees, T.A.; Yu, N.Y.; Ding, J.X.; Liu, C.; Golafshar, M.A.; Rule, W.G.; Vora, S.A.; Ross, H.J.; Ahn, D.H.; et al. Acute Toxicities and Short-Term Patient Outcomes After Intensity-Modulated Proton Beam Radiation Therapy or Intensity-Modulated Photon Radiation Therapy for Esophageal Carcinoma: A Mayo Clinic Experience. Adv. Radiat. Oncol. 2020, 5, 871–879.
- DeCesaris, C.; Berger, M.; Choi, J.I.; Carr, S.R.; Burrows, W.M.; Regine, W.F.; Ii, C.B.S.; Molitoris, J.K. Pathologic complete response (pCR) rates and outcomes after neoadjuvant chemoradiotherapy with proton or photon radiation for adenocarcinomas of the esophagus and gastroesophageal junction. J. Gastrointest. Oncol. 2020, 11, 663–673.
- Lancellotta, V.; Cellini, F.; Fionda, B.; De Sanctis, V.; Vidali, C.; Fusco, V.; Barbera, F.; Gambacorta, M.A.; Corvò, R.; Magrini, S.M.; et al. The role of palliative interventional radiotherapy (brachytherapy) in esophageal cancer: An AIRO (Italian Association of Radiotherapy and Clinical Oncology) systematic review focused on dysphagia-free survival. Brachytherapy 2020, 19, 104–110.
- Dai, Y.; Li, C.; Xie, Y.; Liu, X.; Zhang, J.; Zhou, J.; Pan, X.; Yang, S. Interventions for dysphagia in oesophageal cancer. Cochrane Database Syst. Rev. 2014.
- Bang, Y.-J.; Van Cutsem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 2010, 376, 687–697.
- Gourd, K.; Lai, C.; Reeves, C. ESMO Virtual Congress 2020. Lancet Oncol. 2020, 21, 1403–1404.
- Pech, O.; Pal, S.; Dash, N.R.; Ahuja, V.; Mohanti, B.K.; Vishnubhatla, S.; Sahni, P.; Chattopadhyay, T.K. Palliative Stenting With or Without Radiotherapy for Inoperable Esophageal Carcinoma: A Randomized Trial. J. Gastrointest. Cancer 2010, 43, 63–69.
- Lai, A.; Lipka, S.; Kumar, A.; Sethi, S.; Bromberg, D.; Li, N.; Shen, H.; Stefaniwsky, L.; Brady, P. Role of Esophageal Metal Stents Placement and Combination Therapy in Inoperable Esophageal Carcinoma: A Systematic Review and Meta-analysis. Dig. Dis. Sci. 2018, 63, 1025–1034.
- Liu, B.; Bo, Y.; Wang, K.; Liu, Y.; Tang, X.; Zhao, Y.; Zhao, E.; Yuan, L. Concurrent neoadjuvant chemoradiotherapy could improve survival outcomes for patients with esophageal cancer: A meta-analysis based on random clinical trials. Oncotarget 2017, 8, 20410–20417.
- Zhao, X.; Ren, Y.; Hu, Y.; Cui, N.; Wang, X.; Cui, Y. Neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy for cancer of the esophagus or the gastroesophageal junction: A meta-analysis based on clinical trials. PLOS ONE 2018, 13, e0202185.
- Safran, H.; Winter, K.A.; Wigle, D.A.; DiPetrillo, T.A.; Haddock, M.G.; Hong, T.S.; Leichman, L.P.; Rajdev, L.; Resnick, M.B.; Kachnic, L.A.; et al. Trastuzumab with trimodality treatment for esophageal adenocarcinoma with HER2 overexpression: NRG Oncology/RTOG 1010. J. Clin. Oncol. 2020, 38, 4500.
