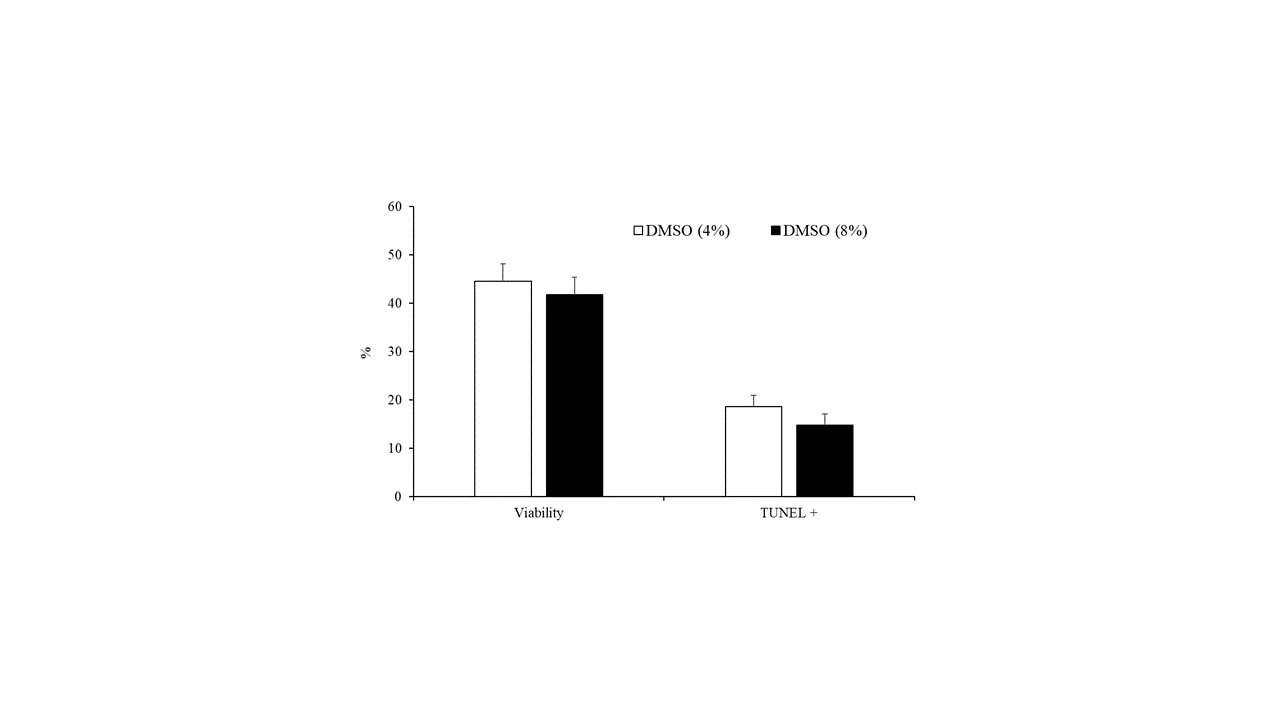The storage of frozen sperm samples from birds in Germoplasm Banks could be a way out for the conservation of thousands of threatened species. The cryopreserved samples could be later used in reintroduction projects. This technology also allows for the selection of individuals of genetic interest and the exchange of samples between distant locations.
Although the sperm cryopreservation is greatly developed in domestic species, in birds it is still in its infancy. The objective of this study was optimize the cryopreservation protocol in a bird of prey, peregrine falcon (Falco peregrinus) which could be used as a model for other threats. Thus, the freezing/thawing protocol and the type and concentration of cryoprotectant were evaluated.
- sperm
- cryopreservation
- birds
- Falcon peregrinus
INTRODUCTION:
At present, the number of species threatened with extinction is above 28,000, of which 14% correspond to avian species [IUCN, 2019]. With fast changes currently occurring in the climate and habitats, thousands of species of wild animals are in danger of disappearing [IUCN, 2019]. A global effort is being made to create a solution for potential emergencies that could lead to the rapid extinction of species. With that in mind, captive breeding and the exchange of individuals between zoos are common practices around the world. However, space is limited, and it is impossible to maintain significant numbers of each existent species. A viable presented solution is the creation of a germplasm bank that contains the sperm, oocytes, or even embryos of the largest possible number of species [Pukazhenthi et al. 2004; Prieto et al. 2014].
Since the storage of eggs or embryos from birds poses a difficult challenge because of their large size and complexity, sperm cells are a good alternative [Long et al. 2013]. Sperm is fairly easy and inexpensive to acquire, and its cryopreservation is much more accessible compared with the oocyte/embryo, because of the small volume of water inside of the sperm [Rosen et al. 2019]. Furthermore, existing technologies allow the semen of many animal species to be collected and used [FAO, 2012]. Frozen semen can be a very important part of ex situ conservation efforts, since it allows for the storage of genetic material from multiple species for a long period of time. Stored semen can be used in reintroduction projects or to enlarge the genetic pool of a fragmented population. It also allows for the selection of individuals of genetic interest and the exchange of samples between distant locations [Brock et al. 1983; Villaverde-Morcillo et al. 2017]. In birds, frozen semen could become an increasingly relevant tool, because captive breeding programs are often slowed down by incompatibility between individuals or by stress, leading to the absence of mating [Bailey et al. 2017]. Cryopreservation of semen is a technique largely used in mammals, but, in birds, there are still some limitations. Avian spermatozoa are filiform shaped, which makes them more susceptible to injury from manipulation [Madeddu et al. 2009]. Additionally, they are highly sensitive to the changes in osmolarity that occur during the freezing/thawing process [Long et al. 2006; Blesbois et al. 2012].
Large numbers of peregrine falcons (Falco peregrinus) are managed in commercial breeding programs and could be used as a model for other birds of prey, especially ones that have spermatozoa with similar characteristics. By studying cryopreservation in falcons, viable solutions can be applied not only to the growing market of falconry, but also to a variety of other birds of prey, including wild endangered ones [Bailey et al. 2017, Al-Daraji et al. 2016].
The objective of this study was to evaluate the effect of different conditions during falcon semen cryopreservation on several sperm characteristics. For this purpose, the freezing protocol (slow freezing in straws vs. fast freezing in pellets), the thawing procedure (37 °C for 30 s vs. 5 °C for 1 min) and the type (DMA vs. DMSO) and concentration of cryoprotectant (3% vs. 8% DMSO) utilized were examined. The ultimate goal was to develop and optimize sperm cryopreservation protocols for this species and to increase the success rate of artificial inseminations with frozen semen.
RESULTS:
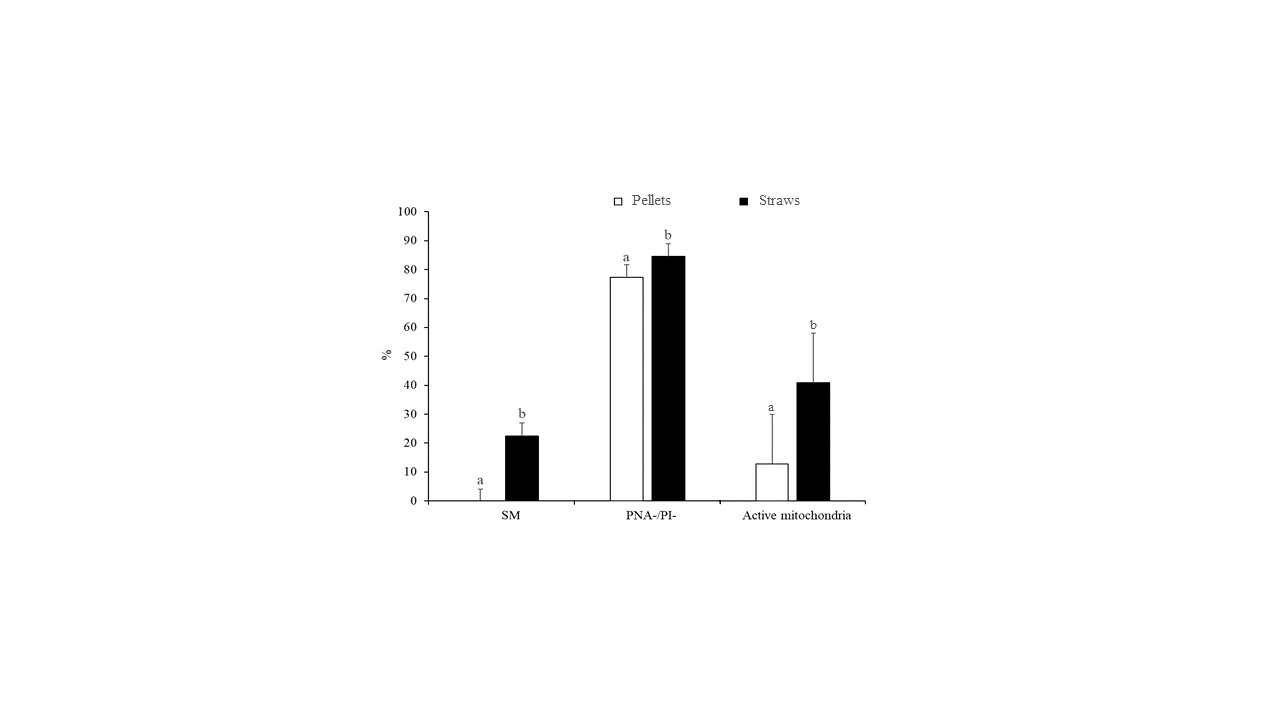
The differences in sperm quality between the two freezing methods (slow freezing in straws and fast freezing in pellets) are indicated in Figure 1. Accordingly, the slow freezing method resulted in greater (p < 0.05) rates of SM (22.5% ± 4.4% vs. 0.0% ± 4.1%), PNA-/PI- (84.6% ± 4.3% vs. 77.4% ± 4.3%), and active mitochondria (41.0% ± 6.7% vs. 12.8% ± 6.7%) compared with ultrarapid freezing (Figure 1).
Figure 1. Characteristics of peregrine falcon sperm cryopreserved by slow freezing in straws and ultrarapid freezing in pellets: motile spermatozoa (SM), viable spermatozoa with intact acrosomes (PNA-/PI-), and spermatozoa with high membrane potential (active mitochondria). Results are expressed as the mean ± S.E.M. a,b Different letters indicate significant differences (p ≤ 0.05) between treatments.
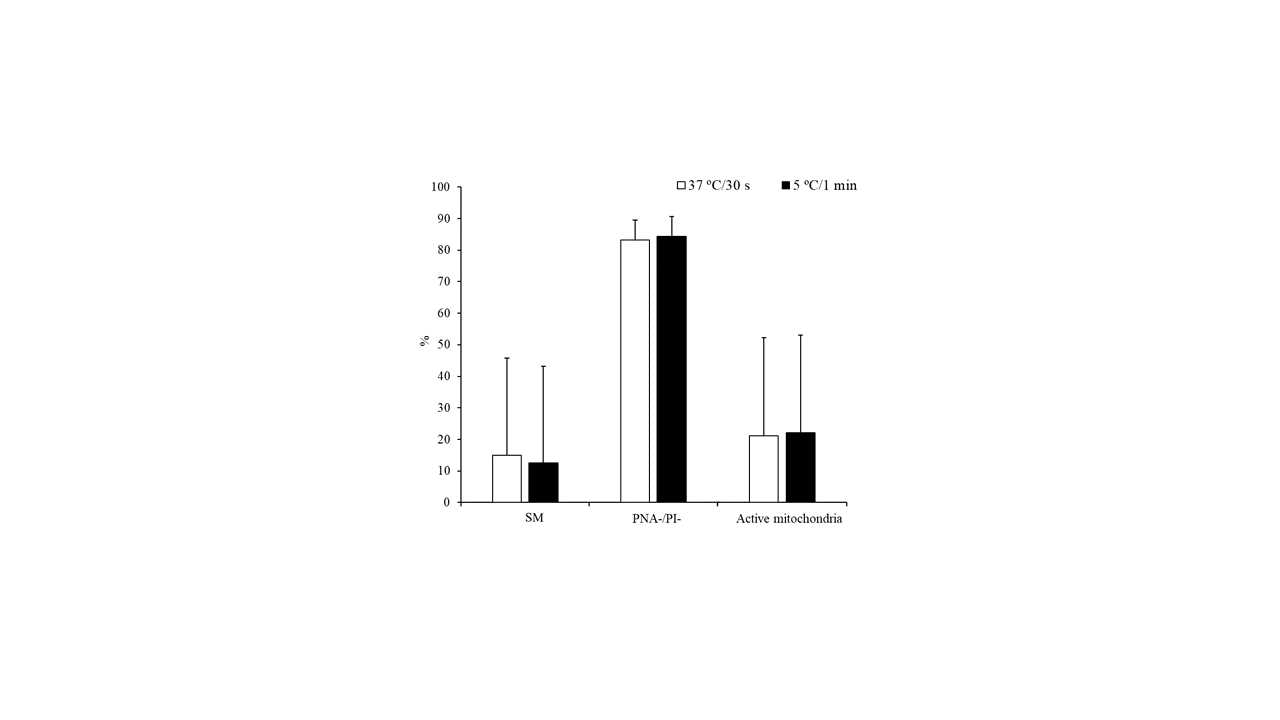
However, no significant differences were detected for the above-mentioned parameters when sperm samples were thawed at 37 °C for 30 s or 5 °C for 1 min (Figure 2).
Figure 2. Characteristics of peregrine falcon sperm thawed at 37 °C for 30 s or 5 °C for 1 min: motile spermatozoa (SM), viable spermatozoa with intact acrosomes (PNA-/PI-), and spermatozoa with high membrane potential (active mitochondria). Results are expressed as the mean ± S.E.M
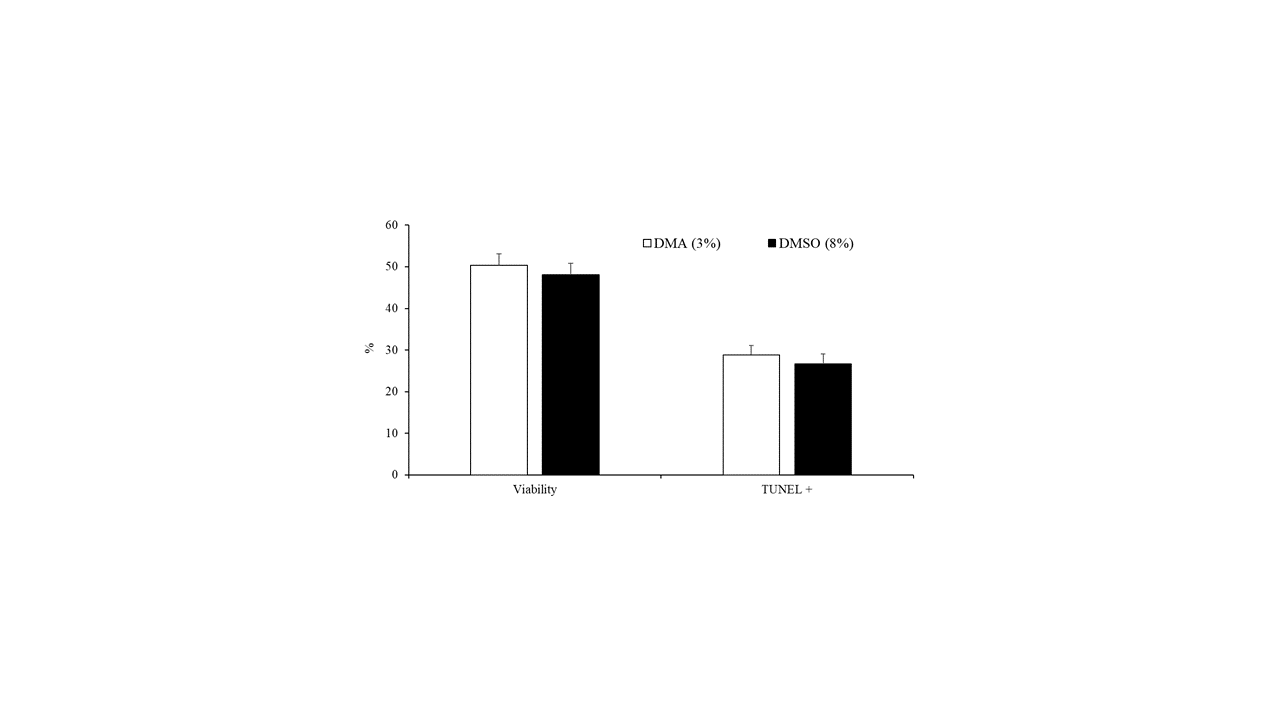
The data on the effect of the type of cryoprotectant (3% DMA or 8% DMSO) on the motility, viability, and DNA fragmentation of peregrine falcon spermatozoa are presented in Table 1 and Figure 3. Ejaculates that were frozen in 8% DMSO had greater values for all motility variables compared with those in 3% DMA (Table 1). Nevertheless, there were no differences (p < 0.05) in the percentage of viable cells and spermatozoa with fragmented DNA between samples frozen with 3% DMA and those frozen in 8% DMSO (Figure 3).
Table 1. Effect of type of cryoprotectant on motility parameters of peregrine falcon spermatozoa.
|
Semen Variables |
DMA 3% |
DMSO 8% |
|
Motile spermatozoa (%) |
4.2 ± 1.9 a |
13.9 ± 1.8 b |
|
Total motility (%) |
7.7 ± 2.3 a |
21.1 ± 2.3 b |
|
Progressive motility (%) |
1.1 ± 0.3 a |
2.1 ± 0.3 b |
|
Straight-line Velocity (VSL; µm/s) |
7.7 ± 1.4a |
12.9 ± 1.4b |
|
Straightness (STR; %) |
49 ± 6.4 a |
70.6 ± 6.2 b |
|
Amplitude of Lateral Head (ALH; µm) |
0.3 ± 0.1 a |
0.8 ± 0.1 b |
|
Beat-cross frequency (BCF; Hz) |
1.2 ± 0.5 a |
3.1 ± 0.5 b |
a,b Different letters within rows reflect significant differences (p < 0.05) among treatments. Results are expressed as the mean ± S.E.M.
Figure 3. Sperm viability and spermatozoa with DNA fragmentation detected by TUNEL assay of samples cryopreserved in DMA (3%) or DMSO (8%). Results are expressed as the mean ± S.E.M.
The concentration of DMSO (4% vs. 8%) did not affect sperm motility parameters (Table 2) or the percentage of viable cells and spermatozoa with fragmented DNA (Figure 4).
Table 2. Effect of DMSO concentration (4% vs. 8%) on sperm motility variables.
|
Semen Variables |
DMSO 4% |
DMSO 8% |
|
Motile spermatozoa (%) |
9.5 ± 1.9 |
8.1 ± 1.9 |
|
Total motility (%) |
14.5 ± 2.7 |
12.6 ± 2.7 |
|
Progressive motility (%) |
2.0 ± 0.5 |
1.6 ± 0.5 |
|
Straight-line Velocity (VSL; µm/s) |
16.1 ± 2.9 |
13.2 ± 2.9 |
|
Straightness (STR; %) |
77.8 ± 10.4 |
69.4 ± 10.4 |
|
Amplitude of Lateral Head (ALH; µm) |
0.4 ± 0.2 |
0.4 ± 0.2 |
|
Beat-cross frequency (BCF; Hz) |
2.5 ± 0.8 |
2.5 ± 0.8 |
Figure 4. Sperm viability and spermatozoa with DNA fragmentation detected by TUNEL assay of samples cryopreserved with different concentrations of DMSO (4% or 8%). Results are expressed as the mean ± S.E.M.
CONCLUSION:
In summary, the freezing method and type of cryoprotectant utilized were identified as critical steps in the process of peregrine falcon sperm cryopreservation. The cryopreservation of peregrine falcon sperm by slow freezing in straws in combination with DMSO resulted in higher motility rates, although the thawing procedure and DMSO concentration had no effect on sperm quality. In addition, the results suggest that DMA is not ideal when slow cooling rates are used. Further artificial insemination studies are required to determine whether fertility rates are greater using slow sperm-freezing methods.
REFERENCES:
- The IUCN Red List of Threatened Species. Available online: https://www.iucnredlist.org/ (accessed on 30 May 2019).
- Pukazhenthi, B.S.; Wildt, D.E. Which reproductive technologies are most relevant to studying, managing and conserving wildlife? Reprod. Fertil. Dev. 2004, 16, 33–46.
- Prieto, M.T.; Sánchez-Calabuig, M.J.; Hildebrandt, T.B.; Saragusty, J. Sperm cryopreservation in wild animals. Eur J. Wildl. Res. 2014, 60, 851–864.
- Long, J. Successful cryopreservation of avian germplasm: Why a multifaceted approach is required. Cryobiology 2013, 67, 406.
- Rosen, M.; Marsh, P.; Quinn, M. Gamete and Embryo Manipulation. In Yen and Jaffe's Reproductive Endocrinology, 8th ed.; Strauss, J.F., Barbieri, R.L., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 823–856.
- FAO. Cryoconservation of Animal Genetic Resources; FAO: Rome, Italy, 2012.
- Brock, M.; Bird, D.; Ansah, G. Cryogenic preservation of spermatozoa of the American kestrel. Int. Zoo Yearb. 1983, 23, 67–71.
- Villaverde-Morcillo, S; Soler, A.J.; Esteso, M.C.; Castaño, C.; Gonzalez, F. Immature and mature sperm morphometry in fresh and frozen-thawed falcon ejaculates. Theriogenology 2017, 98, 94–100.
- Bailey, T.A.; Lierz, M. Veterinary Aspects of Bird of Prey Reproduction. Vet. Clin. N. Am. Exot. Anim. Pract. 2017, 20, 455–483.
- Madeddu, M.; Berlinguer, F.; Ledda, M.; Leoni, G.G.; Satta, V.; Succu, S.; Rotta, A.; Pasciu, V.; Zinellu, A.; Carru, C.; Naitana, S. Ejaculate collection efficiency and post-thaw semen quality in wild-caught Griffon vultures from the Sardinian population. Reprod. Biol. Endocrinol. 2009, 7, 18.
- Long, J.A. Avian Semen Cryopreservation: What Are the Biological Challenges? Poult. Sci. 2006, 85, 232–236.
- Blesbois, E. Biological features of the avian male gamete and their application to biotechnology of conservation. J. Poult. Sci. 2012, 49, 141–149.
- Al-Daraji, H.J.; Al-Shemmary, S.A. Effect of breed of falcon on semen quality traits. Int. J. Conserv. Sci. 2016, 7, 725–734.
This entry is adapted from the peer-reviewed paper 10.3390/ani10040691