Alterations in the Wnt signaling pathway are associated with the advancement of cancers; however, the exact mechanisms responsible remain largely unknown. It has recently been established that heightened intratumoral Wnt signaling correlates with tumor immunomodulation and immune suppression, which likely contribute to the decreased efficacy of multiple cancer therapeutics. Here, we review available literature pertaining to connections between Wnt pathway activation in the tumor microenvironment and local immunomodulation. We focus specifically on preclinical and clinical data supporting the hypothesis that strategies targeting Wnt signaling could act as adjuncts for cancer therapy, either in combination with chemotherapy or immunotherapy, in a variety of tumor types.
- Wnt
- β-catenin
- immunomodulation
- cancer
- immunotherapy
- tumor microenvironment
1. Introduction
2. The Wnt Pathways
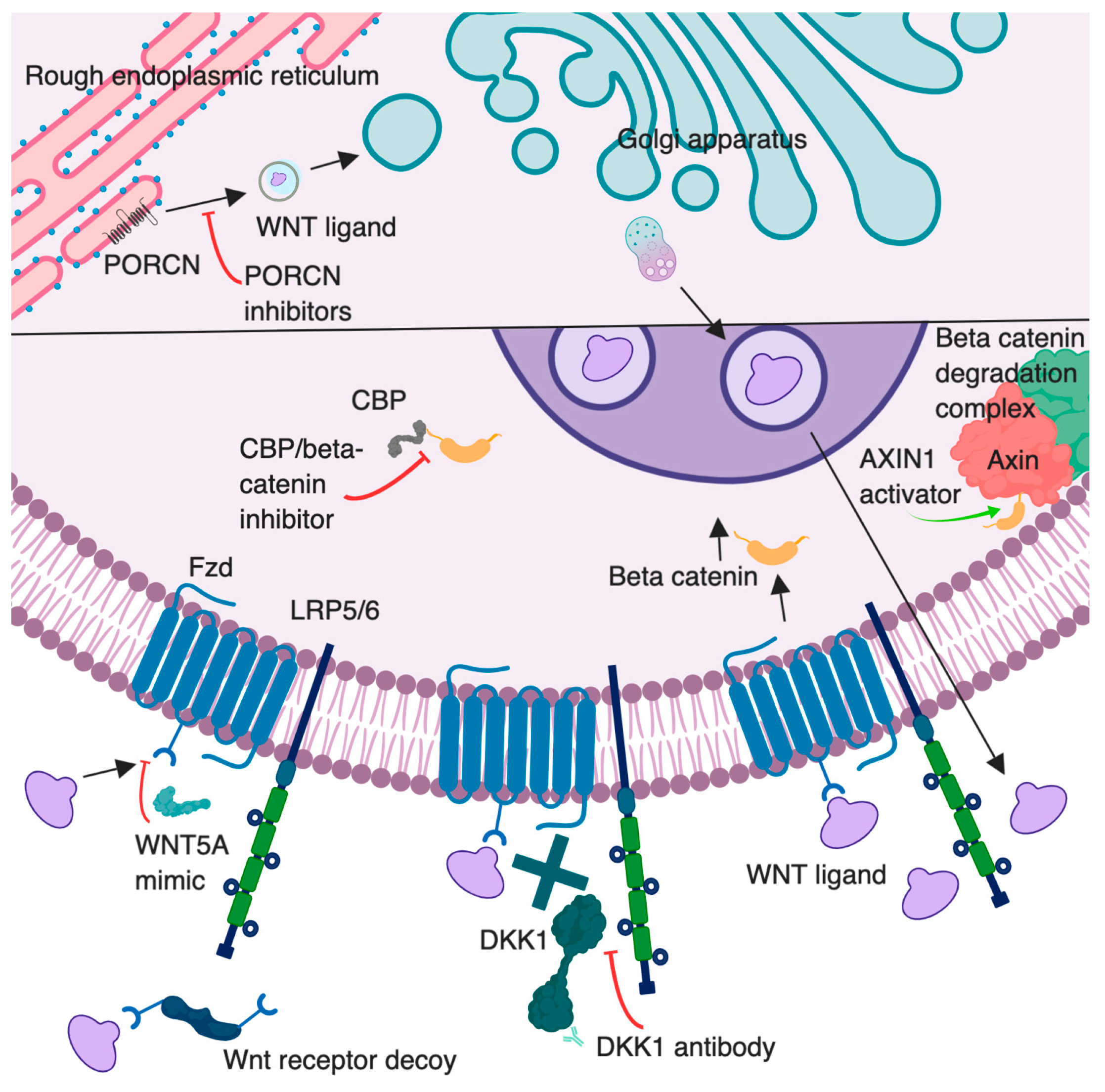
2.1. The Canonical Pathway
2.2. The Noncanonical Pathways
2.3. Wnt Inhibitors
3. Wnt/β-Catenin Signaling and Immunomodulation
3.1. Stem Cells
3.2. Wnt Signaling and Cancer
3.3. Immunity in Cancer
3.4. Wnt Signaling and Leukocyte Differentiation
3.5. Wnt Signaling and Immunomodulation
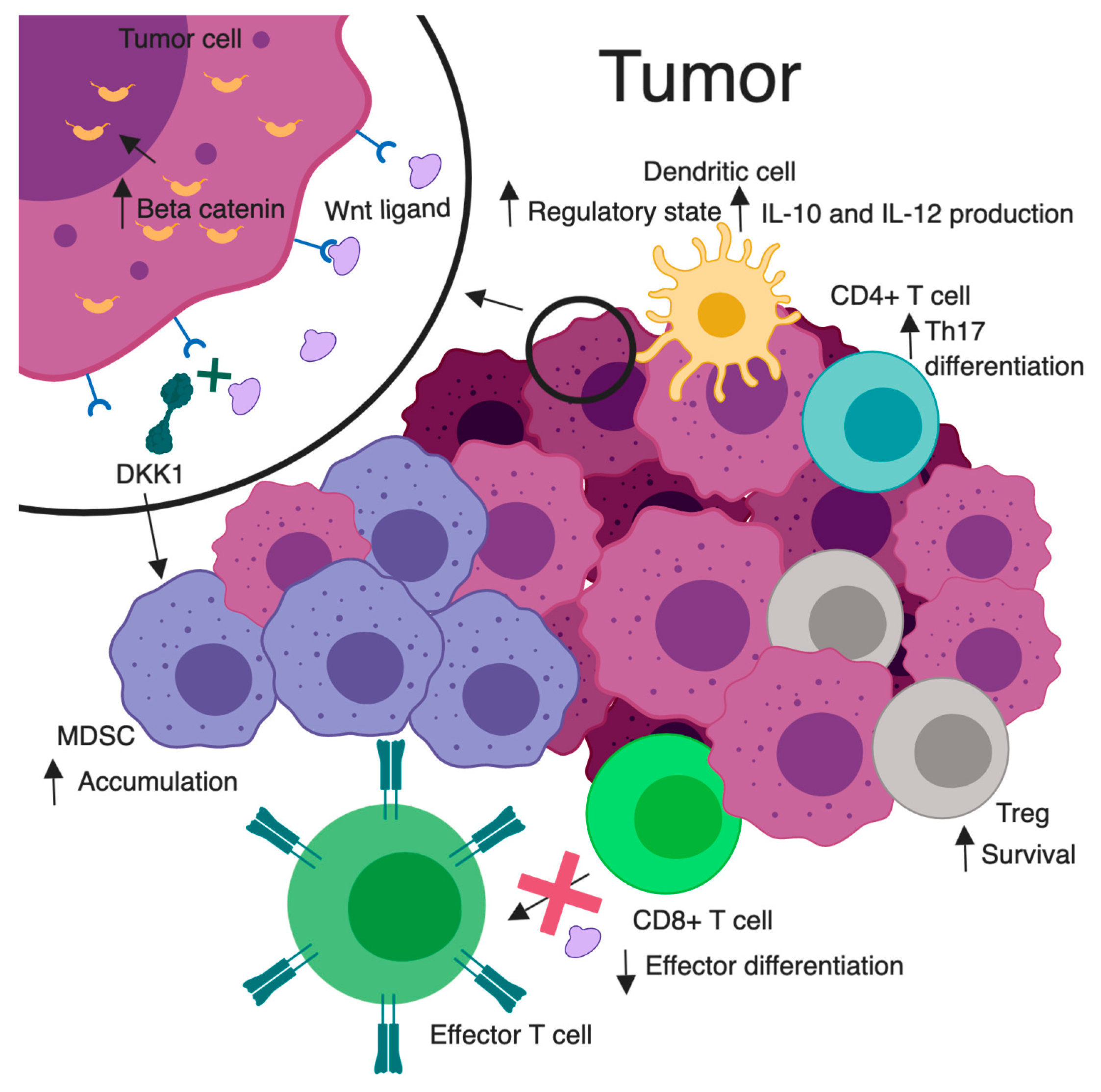
3.6. Immunotherapy
4. Targeting Wnt Signaling as a Novel Therapeutic Option
| Agent | Mechanism of Action | Disease | Phase | Therapy | Identifier |
|---|---|---|---|---|---|
| DKN-01 | DKK1 antibody | Esophageal Neoplasms | 1 | Dose escalation in combination with paclitaxel or pembrolizumab | NCT02013154 |
| Adenocarcinoma of the Gastroesophageal Junction | |||||
| Gastroesophageal Cancer | |||||
| Squamous Cell Carcinoma | |||||
| Gastric Adenocarcinoma | |||||
| DKN-01 | DKK1 antibody | Endometrial Cancer | 2 | Monotherapy or in combination with paclitaxel | NCT03395080 |
| Uterine Cancer | |||||
| Ovarian Cancer | |||||
| DKN-01 | DKK1 antibody | Hepatocellular Carcinoma | 1, 2 | Phase 1/2 as a monotherapy or combination with sorafenib | NCT03645980 |
| DKN-01 | DKK1 antibody | Multiple Myeloma | 1 | Pilot study of combination with lenalidomide/dexamethasone | NCT01711671 |
| DKN-01 | DKK1 antibody | Multiple Myeloma | 1 | Dose escalation | NCT01457417 |
| Solid Tumors | |||||
| Non-Small Cell Lung Cancer | |||||
| DKN-01 | DKK1 antibody | Carcinoma of Intrahepatic and Extra-hepatic Biliary System | 1 | Dose escalation combined with gemcitabine and cisplatin | NCT02375880 |
| Carcinoma of Gallbladder | |||||
| Bile Duct Cancer | |||||
| Cholangiocarcinoma | |||||
| CGX 1321 | PORCN inhibitor | Colorectal Adenocarcinoma | 1 | Single agent dose escalation | NCT03507998 |
| Gastric Adenocarcinoma | |||||
| Pancreatic Adenocarcinoma | |||||
| Bile Duct Carcinoma | |||||
| Hepatocellular Carcinoma | |||||
| Esophageal Carcinoma | |||||
| Gastrointestinal Cancer | |||||
| CGX 1321 | PORCN inhibitor | Solid Tumors | 1 | Single agent dose escalation with or without pembrolizumab | NCT02675946 |
| GI Cancer | |||||
| ETC1922159 | PORCN inhibitor | Solid Tumors | 1 | Single agent dose escalation | NCT02521844 |
| LGK974 | PORCN inhibitor | Pancreatic Cancer | 1 | Single agent and in combination with PDR001 | NCT01351103 |
| BRAF Mutant Colorectal Cancer | |||||
| Melanoma | |||||
| Triple Negative Breast Cancer | |||||
| Head and Neck Squamous Cell Cancer | |||||
| Cervical Squamous Cell Cancer | |||||
| Esophageal Squamous Cell Cancer | |||||
| Lung Squamous Cell Cancer | |||||
| RXC004 | PORCN inhibitor | Cancer | 1 | Dose tolerability | NCT03447470 |
| Solid Tumor | |||||
| Artesunate | Unknown | Hepatocellular Carcinoma | 1 | Single agent dose escalation | NCT02304289 |
| Artesunate | Unknown | Colorectal Cancer | 2 | Neoadjuvant single agent | NCT03093129 |
| Artesunate | Unknown | Solid Tumors | 1 | Single agent dose escalation | NCT02353026 |
| Artesunate | Unknown | Colorectal Cancer | 2 | Neoadjuvant single agent | NCT02633098 |
| Bowel Cancer | |||||
| Artesunate | Unknown | Metastatic Breast Cancer | 1 | Add-on therapy | NCT00764036 |
| Locally Advanced Breast Cancer | |||||
| Niclosamide | AXIN1 activator | Colon Cancer | 1 | Dose escalation | NCT02687009 |
| Niclosamide | AXIN1 activator | Metastatic Prostate Carcinoma | 1 | Dose escalation with enzalutamide | NCT03123978 |
| Recurrent Prostate Carcinoma | |||||
| Stage IV Prostate Cancer | |||||
| Niclosamide | AXIN1 activator | Castration-Resistant Prostate Carcinoma | 1 | Dose escalation with enzalutamide | NCT02532114 |
| Metastatic Prostate Carcinoma | |||||
| Recurrent Prostate Carcinoma | |||||
| Stage IV Prostate Adenocarcinoma | |||||
| Niclosamide | AXIN1 activator | Colorectal Cancer | 2 | Single agent | NCT02519582 |
| Niclosamide | AXIN1 activator | Metastatic Prostate Cancer | 2 | Combination with abirateronae acetate and prednisone | NCT02807805 |
| Recurrent Prostate Cancer | |||||
| Stage IV Prostate Cancer | |||||
| OMP54F28 | Wnt receptor decoy | Hepatocellular Cancer | 1 | Dose escalation with sorafenib | NCT02069145 |
| Liver Cancer | |||||
| OMP54F28 | Wnt receptor decoy | Ovarian Cancer | 1 | Combined with paclitaxel and carboplatin | NCT02092363 |
| OMP54F28 | Wnt receptor decoy | Pancreatic Cancer | 1 | Combined with Nab-paclitaxel and gemcitabine | NCT02050178 |
| Stage IV Pancreatic Cancer | |||||
| OMP54F28 | Wnt receptor decoy | Solid Tumors | 1 | Dose escalation | NCT01608867 |
| Foxy-5 | WNT5A mimic | Metastatic Breast Cancer | 1 | Dose escalation | NCT02020291 |
| Colorectal Cancer | |||||
| Prostate Cancer | |||||
| Foxy-5 | WNT5A mimic | Metastatic Breast Cancer | 1 | Dose escalation | NCT02655952 |
| Metastatic Colon Cancer | |||||
| Metastatic Prostate Cancer | |||||
| PRI724 | CBP/catenin inhibitor | Advanced Pancreatic Cancer | 1 | Dose escalation with gemcitabine | NCT01764477 |
| Metastatic Pancreatic Cancer | |||||
| Pancreatic Adenocarcinoma | |||||
| PRI724 | CBP/catenin inhibitor | Acute Myeloid Leukemia | 1, 2 | Dose escalation, combined with dasatinib for CML or cytarabine for AML | NCT01606579 |
| Chronic Myeloid Leukemia | |||||
| SM08502 | Unknown | Solid Tumors, Adult | 1 | Single agent dose escalation | NCT03355066 |
References
- Wang, B.; Tian, T.; Kalland, K.-H.; Ke, X.; Qu, Y. Targeting Wnt/β-Catenin Signaling for Cancer Immunotherapy. Trends Pharmacol. Sci. 2018, 39, 648–658.
- Galluzzi, L.; Spranger, S.; Fuchs, E.; López-Soto, A. WNT Signaling in Cancer Immunosurveillance. Trends Cell Biol. 2019, 29, 44–65.
- Mora, J.; Mertens, C.; Meier, J.K.; Fuhrmann, D.C.; Brüne, B.; Jung, M. Strategies to Interfere with Tumor Metabolism through the Interplay of Innate and Adaptive Immunity. Cells 2019, 8, 8.
- Sherwood, V. Wnt signaling: An emerging mediator of cancer cell metabolism? Cell. Biol. 2015, 35, 2–10.
- Roel Nusse, H.C. Wnt/b-catenin signaling, disease, and emerging therapeutic modalities. Cell 2017, 169, 985–999.
- Nusse, R.; Varmus, H.E. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell 1982, 31, 99–109.
- Kusserow, A.; Sturm, C.; Hrouda, M.; Lentfer, J.; Schmidt, H.A.; Technau, U.; von Haeseler, A.; Hobmayer, B.; Martindale, M.Q.; Holstein, T.W. Unexpected complexity of the wnt gene familly in a sea anemone. Nature 2005, 433, 156–160.
- Niehrs, C.; Acebron, S.P. Mitotic and mitogenic Wnt signalling. EMBO J. 2012, 31, 2705–2713.
- Rios-Esteves, J.; Resh, M.D. Stearoyl CoA desaturase is required to produce active, lipid-modified Wnt proteins. Cell Rep. 2013, 4, 1072–1081.
- Janda, C.Y.; Waghray, D.; Levin, A.M.; Thomas, C.; Garcia, K.C. Structural basis of Wnt recognition by Frizzled. Science 2012, 337, 59–64.
- Bartscherer, K.; Pelte, N.; Ingelfinger, D.; Boutros, M. Secretion of Wnt Ligands Requires Evi, a Conserved Transmembrane Protein. Cell 2006, 125, 523–533.
- Gross, J.C.; Chaudhary, V.; Bartscherer, K.; Boutros, M. Active Wnt proteins are secreted on exosomes. Nature 2012, 14, 1036–1045.
- Alok, A.; Lei, Z.; Jagannathan, N.S.; Kaur, S.; Harmston, N.; Rozen, S.G.; Tucker-Kellogg, L.; Virshup, D.M. Wnt proteins synergize to activate b-catenin signaling. Cell Sci. 2017, 130, 1532–1544.
- Janda, C.Y.; Dang, L.T.; You, C.; Chang, J.; de Lau, W.; Zhong, Z.A.; Yan, K.S.; Marecic, O.; Siepe, D.; Li, X.; et al. Surrogate wnt agonists that phenocopy canonical wnt and b-catenin signaling. Nature 2017, 545, 234–237.
- Aberle, H.; Bauer, A.; Stappert, J.; Kispert, A.; Kemler, R. beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 1997, 16, 3797–3804.
- Kitagawa, M.; Hatakeyama, S.; Shirane, M.; Matsumoto, M.; Ishida, N.; Hattori, K.; Nakamichi, I.; Kikuchi, A.; Nakayama, K.; Nakayama, K.-I.; et al. An F-box protein, FWD1, mediates ubiquitin-dependent proteolysis of beta-catenin. EMBO J. 1999, 18, 2401–2410.
- Stamos, J.L.; Enos, M.D.; Shah, N.; Weis, W.I. Structural basis of gsk-3 inhibition by n-terminal phosphorylation and by the wnt receptor lrp6. eLife 2014, 3, e01998.
- Behrens, J.; Kuhl, M.; Bruhn, L.; Wedlich, D.; Grosschedl, R.; Birchmeier, W. Functional interaction of beta-catenin with the transcription factor lef-1. Nature 1996, 382, 638–642.
- Molenaar, M.; Van De Wetering, M.; Oosterwegel, M.; Peterson-Maduro, J.; Godsave, S.; Korinek, V.; Roose, J.; Destrée, O.; Clevers, H. XTcf-3 transcription factor mediates beta-catenin-induced axis formation in Xenopus embryos. Cell 1996, 86, 391–399.
- Lustig, B.; Jerchow, B.; Sachs, M.; Weiler, S.; Pietsch, T.; Karsten, U.; Van De Wetering, M.; Clevers, H.; Schlag, P.M.; Birchmeier, W.; et al. Negative Feedback Loop of Wnt Signaling through Upregulation of Conductin/Axin2 in Colorectal and Liver Tumors. Cell. Biol. 2002, 22, 1184–1193.
- De, A. Wnt/ca2+ signaling pathway: A brief overview. Acta Biochim. Biophys. Sin. 2011, 43, 745–756.
- Bryja, V.; Andersson, E.R.; Schambony, A.; Esner, M.; Bryjová, L.; Biris, K.K.; Hall, A.C.; Kraft, B.; Cajanek, L.; Yamaguchi, T.P.; et al. The Extracellular Domain of Lrp5/6 Inhibits Noncanonical Wnt Signaling In Vivo. Biol. Cell 2009, 20, 924–936.
- Stolz, A.; Neufeld, K.; Ertych, N.; Bastians, H. Wnt-mediated protein stabilization ensures proper mitotic microtubule assembly and chromosome segregation. EMBO Rep. 2015, 16, 490–499.
- Cruciat, C.M. Secreted and transmembrane wnt inhibitors and activators. Cold Spring Harb. Perspect. Biol. 2013, 5, a015081.
- Kagey, M.H.; He, X. Rationale for targeting the Wnt signalling modulator Dickkopf‐1 for oncology. J. Pharmacol. 2017, 174, 4637–4650.
- Xiao, Q.; Wang, W.; Chen, S.; Zheng, Y.; Yu, X.; Meeth, K.; Sahraei, M.; Bothwell, A.L.M.; Chen, L.; Bosenberg, M.; et al. Dkk2 imparts tumor immunity evasion through β-catenin-independent suppression of cytotoxic immune-cell activation. Med. 2018, 24, 262–270.
- Hao, H.-X.; Xie, Y.; Zhang, Y.; Charlat, O.; Oster, E.; Avello, M.; Lei, H.; Mickanin, C.; Liu, D.; Ruffner, H.; et al. ZNRF3 promotes Wnt receptor turnover in an R-spondin-sensitive manner. Nature 2012, 485, 195–200.
- Koo, B.-K.; Spit, M.; Jordens, I.; Low, T.Y.; Stange, D.E.; Van De Wetering, M.; Van Es, J.H.; Mohammed, S.; Heck, A.J.R.; Maurice, M.M.; et al. Tumour suppressor RNF43 is a stem-cell E3 ligase that induces endocytosis of Wnt receptors. Nature 2012, 488, 665–669.
- Moffat, L.L.; Robinson, R.E.; Bakoulis, A.; Clark, S.G. The conserved transmembrane RING finger protein PLR-1 downregulates Wnt signaling by reducing Frizzled, Ror and Ryk cell-surface levels in elegans. Development 2014, 141, 617–628.
- Korinek, V.; Barker, N.; Moerer, P.; Van Donselaar, E.; Huls, G.; Peters, P.J.; Clevers, H. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Genet. 1998, 19, 379–383.
- Berge, D.T.; Kurek, D.; Blauwkamp, T.; Koole, W.; Maas, A.; Eroglu, E.; Siu, R.K.; Nusse, R. Embryonic stem cells require Wnt proteins to prevent differentiation to epiblast stem cells. Nature 2011, 13, 1070–1075.
- Andl, T.; Reddy, S.T.; Gaddapara, T.; E Millar, S. WNT signals are required for the initiation of hair follicle development. F1000 Post-Publ. Peer Rev. Biomed. Lit. 2002, 2, 643–653.
- Miao, Y.; Yang, H.; Levorse, J.; Yuan, S.; Polak, L.; Sribour, M.; Singh, B.; Rosenblum, M.D.; Fuchs, E. Adaptive Immune Resistance Emerges from Tumor-Initiating Stem Cells. Cell 2019, 177, 1172–1186.e14.
- Hu, B.; Wang, Q.; Wang, Y.A.; Hua, S.; Sauvé, C.-E.G.; Ong, D.; Lan, Z.D.; Chang, Q.; Ho, Y.W.; Monasterio, M.M.; et al. Epigenetic Activation of WNT5A Drives Glioblastoma Stem Cell Differentiation and Invasive Growth. Cell 2016, 167, 1281–1295.e18.
- Gujral, T.S.; Chan, M.; Peshkin, L.; Sorger, P.K.; Kirschner, M.W.; MacBeath, G. A noncanonical Frizzled2 pathway regulates epithelial-mesenchymal transition and metastasis. Cell 2014, 159, 844–856.
- Zeng, Y.A.; Nusse, R. Wnt Proteins Are Self-Renewal Factors for Mammary Stem Cells and Promote Their Long-Term Expansion in Culture. Cell Stem Cell 2010, 6, 568–577.
- Castagnoli, L.; Cancila, V.; Cordoba-Romero, S.L.; Faraci, S.; Talarico, G.; Belmonte, B.; Iorio, M.V.; Milani, M.; Volpari, T.; Chiodoni, C.; et al. WNT signaling modulates PD-L1 expression in the stem cell compartment of triple-negative breast cancer. Oncogene 2019, 38, 4047–4060.
- Hou, Y.C.; Hsieh, M.H.; Tung, H.L.; Wang, H.C.; Shan, Y.S. Low cd8⁺ t cell infiltration and high pd-l1 expression are associated with level of cd44⁺/cd133⁺ cancer stem cells and predict an unfavorable prognosis in pancreatic cancer. Cancers (Basel) 2019, 11, E541.
- Willert, K.; Danenberg, E.; Duncan, A.W.; Weissman, I.L.; Reya, T.; Yates, J.R., 3rd; Nusse, R. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature 2003, 423, 448–452.
- Sugimura, R.; He, X.C.; Venkatraman, A.; Arai, F.; Box, A.; Semerad, C.; Haug, J.S.; Peng, L.; Zhong, X.-B.; Suda, T.; et al. Noncanonical Wnt Signaling Maintains Hematopoietic Stem Cells in the Niche. Cell 2012, 150, 351–365.
- Nemeth, M.J.; Topol, L.; Anderson, S.M.; Yang, Y.; Bodine, D.M. Wnt5a inhibits canonical wnt signaling in hematopoietic stem cells and enhances repopulation. Natl. Acad. Sci. USA 2007, 104, 15436–15441.
- Cobas, M.; Wilson, A.; Ernst, B.; Mancini, S.J.; MacDonald, H.R.; Kemler, R.; Radtke, F. Beta-catenin is dispensable for hematopoiesis and lymphopoiesis. Exp. Med. 2004, 199, 221–229.
- Jeannet, G.; Scheller, M.; Scarpellino, L.; Duboux, S.; Gardiol, N.; Back, J.; Kuttler, F.; Malanchi, I.; Birchmeier, W.; Leutz, A.; et al. Long-term, multilineage hematopoiesis occurs in the combined absence of beta-catenin and gamma-catenin. Blood 2008, 111, 142–149.
- Gattinoni, L.; Zhong, X.S.; Palmer, D.C.; Ji, Y.; Hinrichs, C.S.; Yu, Z.; Wrzesinski, C.; Boni, A.; Cassard, L.; Garvin, L.M.; et al. Wnt signaling arrests effector t cell differentiation and generates cd8+ memory stem cells. Med. 2009, 15, 808–813.
- Rijsewijk, F.; Van Deemter, L.; Wagenaar, E.; Sonnenberg, A.; Nusse, R. Transfection of the int-1 mammary oncogene in cuboidal RAC mammary cell line results in morphological transformation and tumorigenicity. EMBO J. 1987, 6, 127–131.
- Kinzler, K.W. Lessions from hereditary colorectal cancer. Cell 1996, 87, 159–170.
- Nishisho, I.; Nakamura, Y.; Miyoshi, Y.; Miki, Y.; Ando, H.; Horii, A.; Koyama, K.; Utsunomiya, J.; Baba, S.; Hedge, P. Mutations of chromosome 5q21 genes in FAP and colorectal cancer patients. Science 1991, 253, 665–669.
- Yaeger, R.; Chatila, W.K.; Lipsyc, M.D.; Hechtman, J.F.; Cercek, A.; Sánchez-Vega, F.; Jayakumaran, G.; Middha, S.; Zehir, A.; Donoghue, M.T.; et al. Clinical sequencing defines the genomic landscape of metastatic colorectal cancer. Cancer Cell 2018, 33, 125–136.e3.
- Liu, W.; Mai, M.; Seelan, R.S.; Taniguchi, K.; Krishnadath, K.K.; Halling, K.C.; Cunningham, J.M.; Boardman, L.A.; Qian, C.; Christensen, E.; et al. Mutations in axin2 cause colorectal cancer with defective mismatch repair by activating beta-catenin/tcf signalling. Genet. 2000, 26, 146–147.
- Satoh, S.; Daigo, Y.; Furukawa, Y.; Kato, T.; Miwa, N.; Nishiwaki, T.; Kawasoe, T.; Ishiguro, H.; Fujita, M.; Tokino, T.; et al. AXIN1 mutations in hepatocellular carcinomas, and growth suppression in cancer cells by virus-mediated transfer of AXIN1. Genet. 2000, 24, 245–250.
- Guezguez, B.; Almakadi, M.; Benoit, Y.D.; Shapovalova, Z.; Rahmig, S.; Fiebig-Comyn, A.; Casado, F.L.; Tanasijevic, B.; Bresolin, S.; Masetti, R.; et al. GSK3 Deficiencies in Hematopoietic Stem Cells Initiate Pre-neoplastic State that Is Predictive of Clinical Outcomes of Human Acute Leukemia. Cancer Cell 2016, 29, 61–74.
- Morin, P.J. Activation of beta-Catenin-Tcf Signaling in Colon Cancer by Mutations in beta-Catenin or APC. Science 1997, 275, 1787–1790.
- Rubinfeld, B.; Robbins, P.; El-Gamil, M.; Albert, I.; Porfiri, E.; Polakis, P. Stabilization of beta-Catenin by Genetic Defects in Melanoma Cell Lines. Science 1997, 275, 1790–1792.
- Bodnar, L.; Cierniak, S.; Smoter, M.; Cichowicz, M.; Kozlowski, W.; Szczylik, C.; Wieczorek, M.; Laparska-Przybysz, M. Wnt/b-catenin pathway as a potential prognostic and predictive marker in patients with advanced ovarian cancer. Ovarian Res. 2014, 7, 16.
- Wu, J.; Molin, M.D.; Maitra, A.; de Wilde, R.F.; Wood, L.D.; Eshleman, J.R.; Goggins, M.G.; Wolfgang, C.L.; Canto, M.I.; Schulick, R.D.; et al. Whole-exome sequencing of neoplastic cysts of the pancreas reveals recurrrent mutations in components of ubiquitin-dependent pathways. Natl. Acad. Sci. USA 2011, 108, 21188–21193.
- Assie, G.; Letouzé, E.; Fassnacht, M.; Jouinot, A.; Luscap, W.; Barreau, O.; Omeiri, H.; Rodriguez, S.; Perlemoine, K.; Rene-Corail, F.; et al. Integrated genomic characterization of adrenocortical carcinoma. Genet. 2014, 46, 607–612.
- Schreiber, R.D.; Old, L.J.; Smyth, M.J. Cancer Immunoediting: Integrating Immunity’s Roles in Cancer Suppression and Promotion. Science 2011, 331, 1565–1570.
- Maartje, C.A.; Wouters, F.L.K.; Workel, H.H.; Klip, H.G.; Plat, A.; Kooi, N.M.; Wisman, G.B.A.; Mourits, M.J.E.; Arts, H.J.G.; Oonk, M.H.M.; et al. Treatment regimen, surgical outcome, and t-cell differentiation influence prognostic benefit of tumor-infiltrating lymphocytes in high-grade serous ovarian cancer. Cancer Res. 2016, 22, 714–724.
- Ruan, M.; Tian, T.; Rao, J.; Xu, X.; Yu, B.; Yang, W.; Shui, R. Predictive value of tumor-infiltrating lymphocytes to pathological complete response in neoadjuvant treated triple-negative breast cancers. Pathol. 2018, 13, 66.
- Xiang, P.; Yang, Y.; Sheng, J.; He, Q.; Song, Y.; Yu, W.; Hu, S.; Jin, J. Infiltrating cd4+ t cells attenuate chemotherapy sensitivity in prostate cancer via ccl5 signaling. Prostate 2019, 79, 1018–1031.
- Sato, E.; Olson, S.H.; Ahn, J.; Bundy, B.; Nishikawa, H.; Qian, F.; Jungbluth, A.A.; Frosina, D.; Gnjatic, S.; Ambrosone, C.; et al. Intraepithelial cd8+ tumor-infiltrating lymphocytes and a high cd8+/regulatory t cell ratio are associated with favorable prognosis in ovarian cancer. Natl. Acad. Sci. USA 2005, 102, 18538–18543.
- Thorsson, V.; Brown, S.D.; Wolf, D.; Bortone, D.S.; Yang, T.H.O.; Porta-Pardo, E.; Gao, G.F.; Plaisier, C.L.; Eddy, J.A.; Ziv, E.; et al. The immune landscape of cancer. Immunity 2018, 48, 812–830.
- Xing, S.; Li, F.; Zeng, Z.; Zhao, Y.; Yu, S.; Shan, Q.; Li, Y.; Phillips, F.C.; Maina, P.K.; Qi, H.H.; et al. Tcf1 and lef1 transcription factors establish cd8(+) t cell identity through intrinsic hdac activity. Immunol. 2016, 17, 695–703.
- Steinke, F.C.; Yu, S.; Zhou, X.; He, B.; Yang, W.; Zhou, B.; Kawamoto, H.; Zhu, J.; Tan, K.; Xue, H.H. Tcf-1 and lef-1 act upstream of th-pok to promote the cd4(+) t cell fate and interact with runx3 to silence cd4 in cd8(+) t cells. Immunol. 2014, 15, 646–656.
- Staal, F.J.T.; Luis, T.C.; Tiemessen, M.M. WNT signalling in the immune system: WNT is spreading its wings. Rev. Immunol. 2008, 8, 581–593.
- Xu, Y.; Banerjee, D.; Huelsken, J.; Birchmeier, W.; Sen, J.M. Deletion of beta-catenin impairs t cell development. Immunol. 2003, 4, 1177–1182.
- Jeannet, G.; Boudousquié, C.; Gardiol, N.; Kang, J.; Huelsken, J.; Held, W. Essential role of the Wnt pathway effector Tcf-1 for the establishment of functional CD8 T cell memory. Natl. Acad. Sci. USA 2010, 107, 9777–9782.
- Zhao, D.M.; Yu, S.; Zhou, X.; Haring, J.S.; Held, W.; Badovinac, V.P.; Harty, J.T.; Xue, H.H. Constitutive activation of wnt signaling favors generation of memory cd8 t cells. Immunol. 2010, 184, 1191–1199.
- Yang, Y.; Mlodzik, M. Wnt-Frizzled/Planar Cell Polarity Signaling: Cellular Orientation by Facing the Wind (Wnt). Rev. Cell Dev. Biol. 2015, 31, 623–646.
- Jeevan-Raj, B.; Gehrig, J.; Charmoy, M.; Chennupati, V.; Grandclément, C.; Angelino, P.; Delorenzi, M.; Held, W. The Transcription Factor Tcf1 Contributes to Normal NK Cell Development and Function by Limiting the Expression of Granzymes. Cell Rep. 2017, 20, 613–626.
- Ranheim, E.A.; Kwan, H.C.K.; Wang, Y.-K.; Reya, T.; Weissman, I.L.; Francke, U. Frizzled 9 knock-out mice have abnormal B-cell development. Blood 2005, 105, 2487–2494.
- Yu, Q.; Quinn, W.J., 3rd; Salay, T.; Crowley, J.E.; Cancro, M.P.; Sen, J.M. Role of beta-catenin in b cell development and function. Immunol. 2008, 181, 3777–3783.
- Zhou, J.; Cheng, P.; Youn, J.-I.; Cotter, M.J.; Gabrilovich, D.I. Notch and Wingless Signaling Cooperate in Regulation of Dendritic Cell Differentiation. Immunology 2009, 30, 845–859.
- Sato, N.; Yamabuki, T.; Takano, A.; Koinuma, J.; Aragaki, M.; Masuda, K.; Ishikawa, N.; Kohno, N.; Ito, H.; Miyamoto, M.; et al. Wnt Inhibitor Dickkopf-1 as a Target for Passive Cancer Immunotherapy. Cancer Res. 2010, 70, 5326–5336.
- Kimura, H.; Fumoto, K.; Shojima, K.; Nojima, S.; Osugi, Y.; Tomihara, H.; Eguchi, H.; Shintani, Y.; Endo, H.; Inoue, M.; et al. CKAP4 is a Dickkopf1 receptor and is involved in tumor progression. Clin. Investig. 2016, 126, 2689–2705.
- D’Amico, L.; Mahajan, S.; Capietto, A.-H.; Yang, Z.; Zamani, A.; Ricci, B.; Bumpass, D.B.; Meyer, M.; Su, X.; Wang-Gillam, A.; et al. Dickkopf-related protein 1 (Dkk1) regulates the accumulation and function of myeloid derived suppressor cells in cancer. Exp. Med. 2016, 213, 827–840.
- Qian, J.; Zheng, Y.; Zheng, C.; Wang, L.; Qin, H.; Hong, S.; Li, H.; Lu, Y.; He, J.; Yang, J.; et al. Active vaccination with dickkopf-1 induces protective and therapeutic antitumor immunity in murine multiple myeloma. Blood 2012, 119, 161–169.
- Valencia, J.; Hidalgo, L.; Hernandez-Lopez, C.; Canseco, N.M.; Vicente, A.; Varas, A.; Sacedon, R. Wnt5a signaling increases il-12 secretion by human dendritic cells and enhances ifn-gamma production by cd4+ t cells. Lett. 2014, 162, 188–199.
- Sato, A.; Kayama, H.; Shojima, K.; Matsumoto, S.; Koyama, H.; Minami, Y.; Nojima, S.; Morii, E.; Honda, H.; Takeda, K.; et al. The wnt5a-ror2 axis promotes the signaling circuit between interleukin-12 and interferon-gamma in colitis. Rep. 2015, 5, 10536.
- Keerthivasan, S.; Aghajani, K.; Dose, M.; Molinero, L.; Khan, M.W.; Venkateswaran, V.; Weber, C.; Emmanuel, A.O.; Sun, T.; Bentrem, D.J.; et al. Beta-catenin promotes colitis and colon cancer through imprinting of proinflammatory properties in t cells. Transl. Med. 2014, 6, 225ra228.
- Ding, Y.; Shen, S.; Lino, A.C.; Lafaille, M.A.C.D.; Lafaille, J.J. Beta-catenin stabilization extends regulatory T cell survival and induces anergy in nonregulatory T cells. Med. 2008, 14, 162–169.
- Hong, Y.; Manoharan, I.; Suryawanshi, A.; Majumdar, T.; Angus-Hill, M.L.; Koni, P.A.; Manicassamy, B.; Mellor, A.L.; Munn, D.H.; Manicassamy, S. Beta-catenin promotes regulatory t-cell responses in tumors by inducing vitamin a metabolism in dendritic cells. Cancer 2015, 75, 656–665.
- Hong, Y.; Manoharan, I.; Suryawanshi, A.; Shanmugam, A.; Swafford, D.; Ahmad, S.; Chinnadurai, R.; Manicassamy, B.; He, Y.; Mellor, A.L.; et al. Deletion of lrp5 and lrp6 in dendritic cells enhances antitumor immunity. Oncoimmunology 2016, 5, e1115941.
- Baur, A.S.; Lutz, M.B.; Schierer, S.; Beltrame, L.; Theiner, G.; Zinser, E.; Ostalecki, C.; Heidkamp, G.; Haendle, I.; Erdmann, M.; et al. Denileukin diftitox (ONTAK) induces a tolerogenic phenotype in dendritic cells and stimulates survival of resting Treg. Blood 2013, 122, 2185–2194.
- Yaguchi, T.; Goto, Y.; Kido, K.; Mochimaru, H.; Sakurai, T.; Tsukamoto, N.; Kudo-Saito, C.; Fujita, T.; Sumimoto, H.; Kawakami, Y. Immune suppression and resistance mediated by constitutive activation of wnt/beta-catenin signaling in human melanoma cells. Immunol. 2012, 189, 2110–2117.
- Fu, C.; Liang, X.; Cui, W.; Ober-Blobaum, J.L.; Vazzana, J.; Shrikant, P.A.; Lee, K.P.; Clausen, B.E.; Mellman, I.; Jiang, A. Beta-catenin in dendritic cells exerts opposite functions in cross-priming and maintenance of cd8+ t cells through regulation of il-10. Natl. Acad. Sci. USA 2015, 112, 2823–2828.
- Luke, J.J.; Bao, R.; Sweis, R.F.; Spranger, S.; Gajewski, T.F. Wnt/beta-catenin pathway activation correlates with immune exclusion across human cancers. Cancer Res. 2019, doi:10.1158/1078-0432.CCR-18-1942.
- Spranger, S.; Bao, R.; Gajewski, T.F. Melanoma-intrinsic beta-catenin signalling prevents anti-tumour immunity. Nature 2015, 523, 231–235.
- Nsengimana, J.; Laye, J.; Filia, A.; O’Shea, S.; Muralidhar, S.; Pozniak, J.; Droop, A.; Chan, M.; Walker, C.; Parkinson, L.; et al. Beta-catenin-mediated immune evasion pathway frequently operates in primary cutaneous melanomas. Clin. Investig. 2018, 128, 2048–2063.
- Massi, D.; Romano, E.; Rulli, E.; Merelli, B.; Nassini, R.; De Logu, F.; Bieche, I.; Baroni, G.; Cattaneo, L.; Xue, G.; et al. Baseline beta-catenin, programmed death-ligand 1 expression and tumour-infiltrating lymphocytes predict response and poor prognosis in braf inhibitor-treated melanoma patients. J. Cancer 2017, 78, 70–81.
- Taylor, A.; Rothstein, D.; Rudd, C.E. Small-molecule inhibition of pd-1 transcription is an effective alternative to antibody blockade in cancer therapy. Cancer Res. 2018, 78, 706–717.
- Li, C.-W.; Lim, S.-O.; Xia, W.; Lee, H.-H.; Chan, L.-C.; Kuo, C.-W.; Khoo, K.-H.; Chang, S.-S.; Cha, J.-H.; Kim, T.; et al. Glycosylation and stabilization of programmed death ligand-1 suppresses T-cell activity. Commun. 2016, 7, 12632.
- Simsek, M.; Bilici, M. Immunological agents used in cancer treatment. Eurasian J. Med. 2019, 1, 90–94.
- Hoos, A. Development of immuno-oncology drugs—From CTLA4 to PD1 to the next generations. Rev. Drug Discov. 2016, 15, 235–247.
- Cortés, J.; André, F.; Gonçalves, A.; Kümmel, S.; Martín, M.; Schmid, P.; Schuetz, F.; Swain, S.M.; Easton, V.; Pollex, E.; et al. IMpassion132 Phase III trial: Atezolizumab and chemotherapy in early relapsing metastatic triple-negative breast cancer. Oncol. 2019, doi:10.2217/fon-2019-0059.
- Wang, C.; Kulkarni, P.; Salgia, R. Combined Checkpoint Inhibition and Chemotherapy: New Era of 1st-Line Treatment for Non-Small-Cell Lung Cancer. Ther. Oncolytics 2019, 13, 1–6.
- Weiss, S.A.; Wolchok, J.D.; Sznol, M. Immunotherapy of Melanoma: Facts and Hopes. Cancer Res. 2019, doi:10.1158/1078-0432.CCR-18-1550.
- Wolchok, J.D.; Gonzalez, R.; Rutkowski, P.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Wagstaff, J.; Schadendorf, D.; Ferrucci, P.F.; Smylie, M.; et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. Engl. J. Med. 2017, 377, 1345–1356.
- Galluzzi, L.; Yamazaki, T.; Kroemer, G. Linking cellular stress responses to systemic homeostasis. Rev. Mol. Cell Biol. 2018, 19, 731–745.
- Spranger, S.; Luke, J.J.; Bao, R.; Zha, Y.; Hernandez, K.M.; Li, Y.; Gajewski, A.P.; Andrade, J.; Gajewski, T.F. Density of immunogenic antigens does not explain the presence or absence of the T-cell–inflamed tumor microenvironment in melanoma. Natl. Acad. Sci. USA 2016, 113, E7759–E7768.
- Lu, B.; Green, B.A.; Farr, J.M.; Lopes, F.C.; Van Raay, T.J.; Lo, H.-W. Wnt Drug Discovery: Weaving Through the Screens, Patents and Clinical Trials. Cell. Basis Metastasis Road Ther. 2016, 8, 82.
- Seth, C.; Altaba, A.R. Metastases and Colon Cancer Tumor Growth Display Divergent Responses to Modulation of Canonical WNT Signaling. PLoS ONE 2016, 11, e0150697.
- Holtzhausen, A.; Zhao, F.; Evans, K.S.; Tsutsui, M.; Orabona, C.; Tyler, D.S.; Hanks, B.A. Melanoma-derived wnt5a promotes local dendritic-cell expression of ido and immunotolerance: Opportunities for pharmacologic enhancement of immunotherapy. Cancer Immunol. Res. 2015, 3, 1082–1095.
- Arqués, O.; Puig, I.; Tenbaum, S.P.; Argilés, G.; Dienstmann, R.; Fernández, N.; Caratù, G.; Matito, J.; Silberschmidt, D.; Rodon, J.; et al. Tankyrase inhibition blocks wnt/β-catenin pathway and reverts resistance to pi3k and akt inhibitors in the treatment of colorectal cancer. Cancer Res. 2016, 22, 644–656.
- Fischer, M.M.; Cancilla, B.; Yeung, V.P.; Cattaruzza, F.; Chartier, C.; Murriel, C.L.; Cain, J.; Tam, R.; Cheng, C.-Y.; Evans, J.W.; et al. WNT antagonists exhibit unique combinatorial antitumor activity with taxanes by potentiating mitotic cell death. Adv. 2017, 3, e1700090.
- S. National Library of Medicine. Clnicaltrial.Gove. Available online: https://clinicaltrials.gov/ct2/home (accessed on 30 January 2019).
- Li, L.N.; Zhang, H.D.; Yuan, S.J.; Tian, Z.Y.; Wang, L.; Sun, Z.X. Artesunate attenuates the growth of human colorectal carcinoma and inhibits hyperactive wnt/beta-catenin pathway. J. Cancer 2007, 121, 1360–1365.
- Ahn, S.Y.; Kim, N.H.; Lee, K.; Cha, Y.H.; Yang, J.H.; Cha, S.Y.; Cho, E.S.; Lee, Y.; Cha, J.S.; Cho, H.S.; et al. Niclosamide is a potential therapeutic for familial adenomatosis polyposis by disrupting Axin-GSK3 interaction. Oncotarget 2017, 8, 31842–31855.
- Jimeno, A.; Chugh, R.; Dupont, J.; Uttamsingh, S.; Kapoun, A.M.; Smith, D.C.; Messersmith, W.; Stagg, R.; Xu, L.; Brachmann, R.K.; et al. A First-in-Human Phase I Study of the Anticancer Stem Cell Agent Ipafricept (OMP-54F28), a Decoy Receptor for Wnt Ligands, in Patients with Advanced Solid Tumors. Cancer Res. 2017, 23, 7490–7497.
- Canesin, G.; Evans-Axelsson, S.; Hellsten, R.; Krzyzanowska, A.; Prasad, C.P.; Bjartell, A.; Andersson, T. Treatment with the WNT5A-mimicking peptide Foxy-5 effectively reduces the metastatic spread of WNT5A-low prostate cancer cells in an orthotopic mouse model. PLoS ONE 2017, 12, e0184418.
- Emami, K.H.; Nguyen, C.; Ma, H.; Kim, D.H.; Jeong, K.W.; Eguchi, M.; Moon, R.T.; Teo, J.L.; Kim, H.Y.; Moon, S.H.; et al. A small molecule inhibitor of beta-catenin/creb-binding protein transcription [corrected]. Natl. Acad. Sci. USA 2004, 101, 12682–12687.
