Nutritional quality and the well-being of the body system are directly linked aspects of human survival. From the unborn foetus to adulthood, the need for sustainable access to micronutrient-rich foods is pertinent and the global consumption of banana and plantain fruits, in effect, contributes to the alleviation of the scourge of malnutrition. This review is particularly aimed at evaluating the pharmacological dimensions through the biological mechanisms of Musa fruits in the body, which represent correlations with their constituent micronutrient factors and dietary polyphenolic constituents such as minerals, vitamin members, anthocyanins, lutein, α-,β- carotenes, neoxanthins and cryptoxanthins, epi- and gallo catechins, catecholamines, 3-carboxycoumarin, β-sitosterol, monoterpenoids, with series of analytical approaches for the various identified compounds being highlighted therein.
- biomechanism
- chronic diseases
- dietary compounds
- medicinal plants
- musa
- micronutrients
- secondary metabolites
1. Introduction
Micronutrient deficiency has developed into a key issue in developing and third world nations, with more than two billion people affected globally and expectant mothers and children being the majorly affected group[1][2]. Infants require close monitoring because they are in the sensitive growth and development phase of life [3]. Micronutrient deficiencies have negative health effects such as retardation of growth and development, compromised immunological system, weakened neuromotor efficiency and ultimately mortality[4]. Furthermore, micronutrient deficiencies result in lowered intelligence quotient levels, reduced work capacity and lower earning power[5][6]. Micronutrient deficiency is essentially a latent form of hunger which sets in due to disruption of absorption processes as a result of infection or disease conditions[7]. Poverty is a focal factor in low- and middle-income nations and it deepens the burden of micronutrient deficiency, just as poor dietary patterns (low intake of fruits) in the lower social class of developing countries are equally a liable factor [6][8]. Fruits offer chemopreventive potentials against malignant cancerous cells due to their biochemical constitution[9][10][11]. Population-based studies have indicated an inverse relationship between fruit consumption with cancer mortality and its recurrence [11][12][13]. Dietary administration of fruits has also been reported to be effective in the inhibition and chemoprevention of tumor malignancy [14]. Several studies have also identified the chemopreventive potential of fruit juices against cancerous cell lines[14][15][16]. An inverse association has been identified with fruit micronutrients and deposition of fat and cardiovascular disease among obese populations[17][18][19]. Similarly, epidemiological studies widely indicate a dose-related link between fruit intake and chronic diseases[20][21][22][23][24]. In relation to the forgoing discourse, a number of fresh fruits were surveyed and obtained from supermarkets, and are shown in Figure 1.
Figure 1. Photos of commonly consumed fruits including M. sinensis and M. paradisiaca.
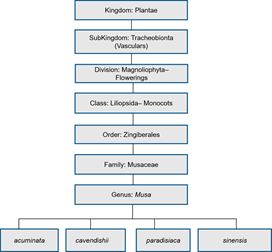
Figure 2. Scientific classification of Musa genus.
The Musa plants range from two to nine metres in height (cultivated) and 10–15 m (wild), consisting of the false stem (pseudostem), corm, foliar including the flowering part. Banana (M. sinensis L.) is a tree-like, perennial herb. The term banana was developed out of “banan” of Arab origin (i.e., “finger” in translation)[25][26] [26]. It was introduced to parts of western Africa (Guinea) from Portugal, with the first domestication of banana in South-eastern Asia[27]. The plantain (M. paradisiaca L.), similar to the unripe banana, is larger, has a starchy flesh and can be used in the unripe and cooked forms. It is a tropical staple, ranked the tenth most important in the world and contains more starch and less sugar than a banana. The mature, yellow variety can be peeled as is typically done with banana.
The Banana family, broadly used to describe the Musa genus and its herbaceous members, including the fruits they bear, have flowers that are medicinally useful in the treatment of bronchitis, dysentery (gastro-intestinal infection bowel movement) and ulcers. Diabetic patients are traditionally served cooked flowers, while the astringent sap is usually applied for conditions such as hysteria, epileptic seizures, fever, leprosy and diarrhoea[28]. It is also useful in cases of haemorrhoids, bites and stings. Young leaves are applied as poultices on burns and other skin disorders, while the leaf and unripe peel ashes are taken for digestive conditions like diarrhoea and dysentery including the treatment of pernicious ulcers. In India, roots and seed mucilage are administered to ameliorate disorders of the digestive system[28]. Banana peel protects the fruit, contributes a minimum of 30% to the total weight of the banana and contains substantial quantities of phosphorous and nitrogen[29]. It also supplies key nutritional mineral elements like magnesium and potassium[30], and contains higher phytochemical constituent in the peel than the flesh (pulp)[31]. In sub-Saharan Africa, more than 30 million people are estimated to feed on bananas as their main energy source as most African breeders and growers still operate at the subsistent level. There is a rising investigative pattern in the use of unripe banana products by consumers for their polyphenol content[32], anthocyanin in the flesh[33] and antioxidative potential in unripe banana flour[34][35]. Studies from literature reveal that non-commercial cultivars have higher levels of antioxidative propensity[36][37]. Plantain is reliably a year-round staple, especially in third world nations laden with inadequate technologies in the frontiers of storing, preserving and transporting food products. On the African continent, plantain fruits meet about one-quarter of the carbohydrate requirements of 70 million people thereabout. It is a versatile culinary raw material for products like chips or dodo (baked or roasted), fufu, porridge, flour eaten with soup, or eaten alone depending on the consumer’s taste[38]. Banana and plantain fruits are quite rich in dietary fibres, which is essential for the optimal functioning of the gastro-intestinal and digestive system [30][39]. Studies have reported higher nutritional profiles in Musa fruit peels compared to a number of fruit peels such as Mangifera indica, Carica papaya, Citrus sinensis, Ananas comosus, Malus domestica, Citrullus lanatus and Punica granatum [40]. A detailed comparative profile of nutritional factors with regards to the aforementioned is indicated in Table 1.
Table 1. Comparative nutritional profile of fruit peels of Musa with other fruits.
| Nutritional Factors | Other Fruit Peels (g/100 g) |
|---|---|
| Protein | > Pineapple, Mango, Orange, Apple, Pomegranate. |
| Carbohydrate | > Pawpaw, Watermelon |
| Ash content | > Pawpaw, Pineapple, Mango, Apple, Orange, Pomegranate, Watermelon |
| Calcium | > Pawpaw, Pineapple, Apple, Watermelon |
| Iron | > Mango, Pomegranate |
| Zinc | > Mango, Apple, Pomegranate |
| Manganese | > Pawpaw, Pineapple, Apple, Orange, Pomegranate, Watermelon. |
Source: [40].
2. Micronutrients
They broadly encompass trace elements, vitamins and mineral elements key to regular cellular and molecular functioning, which makes them of significance to human health in spite of their small requirement levels[41][42][43]. Micronutrient deficiency alleviation is vital to the prevention of chronic disease and mortality amongst deficient populations[44][45]. A number of micronutritional constituents have been identified in the compartments of Musa fruits as shown in Table 2.
Table 2. Micronutritional factors identified in fruit compartments of Musa species fruits including the analytical methods.
| Micronutrients | Soft flesh (Pulp) | Peel |
|---|---|---|
| Manganese | Musa spp. [46][AAS- Atomic Absorption Spectrophotometry] | M. paradisiaca[47] [AAS-Atomic Absorption Spectrophotometry] |
| Zinc | Musa spp. [48] [C18RP-HPLC; Microtitre Plate Spectrophotometry; Inductively Coupled Plasma- Optical Emission Spectrometry ICP-OES] | |
| Iron | Musa spp.[48] [C18RP-HPLC; MicrotitrePlate Spectrophotometry; ICP-OES] | Musa spp.[48] [C18RP-HPLC; Microtitre Plate Spectrophotometry;ICP-OES] |
| Copper | M. paradisiaca[49] | |
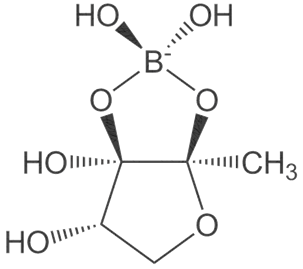
| Boron | Musa (3.72 mg/kg) [50] [Neutron γ-ray activation analysis] | |
| Phosphorus | M. sinensis and M. paradisiaca[39][ICP-OES] | M. sinensis and M. paradisiaca [39][51] [ICP-OES] |
| Thiamine, Riboflavin, Niacin, Folate, Pantothenic acid and Pyridoxine | Banana and Plantain (Musa spp.)[32] |
3. Biomechanismal Implications of Micronutritional Factors of Musa Species Fruits
3.1. Antioxidant Mechanism
Zinc, structurally depicted in Figure 3, is involved in cellular proliferation, differentiation and apoptosis. It prevents the formation of free radicals and cushions the side effects of anti-inflammatory mechanisms [52][53][54]. Zinc deficiency increases inflammatory cytokine levels, oxidative stress as well as cellular dysfunction[55]. Zinc exhibits its antioxidant capacity through the induction and inhibition of hemeoxygenase and NADPH oxidase respectively[56]. Similarly, zinc has been reported to deplete biomarkers of oxidative stress on the basis of human mononuclear cell experiments[56]. It is also involved in the upward regulation of the gene expressions which encode for antioxidant and detoxification molecules[55]. The antioxidant effect of zinc has also been identified in the mechanism of protein sulfhydryl stabilization which is targeted at nullifying oxidation[55]. Zinc protects the cells of the endothelium from reactive oxygen species like hydrogen peroxide through the biological synthesis of glutathione which is stimulated by the Nrf2 (nuclear erythroid 2) factor [57].
Figure 3. Zinc structure.
3.2. Blood Sugar Balance and Immunity
Dietary zinc is involved in the synthesis and secretion processes of insulin within the β-cells of the pancreas, on the basis of the β-cell viability role of zinc in an animal model[58], while it contributes to immunity by mediating in infection resistance and activating the T lymphocyte[58][59]. Animal experiments have confirmed the role of zinc in immunological memory on the basis of antibody recall reactions to T-dependent and independent antigens [60][61].
3.3. Neurological Activity
Dietary zinc acts as a neurological transmitter in the brain and is functionally key in cognition, learning and memory[62] and activates the neuron-based Erk signal mechanism[63]. The regulation of memory formation has been depicted by zinc via the Erk pathway[64].
3.4. Immunological, Neurological, Antioxidant and Anticancer Mechanism
Dietary boron, structurally shown in Figure 4 is biologically essential in a number of modes such as improved central nervous system and enhanced immunity. A number of in vivo and human studies have pointed out that dietary boron is functional in the activity of the brain with its deficiency being associated with a drop-in electrical activity [65][66]. Furthermore, dietary boron deprivation studies have indicated reduced high-frequency and a spiked low-frequency electric activity in the brain[66]. In the same vein, low boron intake (< 0.3 mg/d) assessments in human models reflected lowered cognitive-motor functions. It has been reported from in vivo investigations that boron has the capacity to reverse oxidative stress and free radical production usually triggered by endotoxins. Furthermore, the antioxidative stress capacity of boron has been identified via β-cell preservation in the pancreas, including a dose-dependent pattern (5–20 mg/kg) in tissue damage amelioration [66]. In a similar mode, boron mitigates oxidative stress by increasing the oxidant-neutralizing glutathionic reserves [67]. The induction of boron reverses the oxidative stress expressed in cellular carcinoma of the hepatic system[68]. It also enhances metabolism in the hepatic system[69] and functions collaboratively with calcium, magnesium and phosphorus in the regulation of parathyroid function[70]. Anticancer inhibition against proliferative cell death in prostate and breast cancer cell lines (LNCaP, DU-145) have been reported in boric acid[71][72][73]. Furthermore, the non-tumor prostate cell lines (RWPE-1, PWR-1E) were inhibited in a dose-dependent form (100–1000 µM) by boric acid, while the PC-3 cancer cell line was experimentally inhibited at higher levels than the observed blood levels[71]. There is also the antiproliferative mechanism of boric acid identified in a dose-dependent (500–1000 µM) depletion of MAPK proteins [73].
Figure 4. Boron structure.
3.5. Antioxidant Mechanism
Iron, depicted structurally in Figure 5, is an essential building block for red blood cell haemoglobin which invariably contributes to oxygen distribution from the lungs [74]. Iron deficiency is a very common nutrient disorder that causes anaemia. Iron is important in preventing the susceptibility of the placental unit of foetus from oxidative stress[75]. There is a body of thought that projects the antioxidant mechanism of micronutrients as oxidative stress reducers which enables improved antenatal and postpartum conditions[75][76].
Figure 5. Iron structure.
3.6. Blood Sugar Balance and Antioxidant Activity
Manganese deficiency is not quite pronounced in humans as opposed to animals[77]. However, a significant chunk is lost in refined foods and so fruit intake presents a rich source of dietary manganese[78]. Manganese enhances blood sugar balance as a metalloenzyme constituent implicated in the synthesis of metabolic processes for glucose and glutamine [78]. It also exhibits antioxidant capacity as a component of the superoxide dismutase (SOD) which combats free radicals [81,82] and is significant because of its focal mitochondrial location in the protection of DNA and genetic make-up. Manganese is thus an important biochemical entity and its molecular structure shown in Figure 6.
Figure 6. Manganese structure.
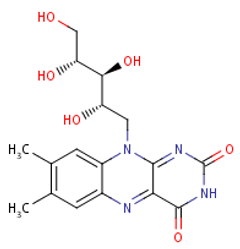
Folate (Vitamin B9) enables red blood cell production (erythropoiesis). It enhances immunity levels and biosynthesizes nucleic acids, nerve tissues (brain functionality) and proteins[79][80]. It helps in DNA damage risk prevention and immunological homeostasis[81][82][83]. In addition, folate has epileptogenic (anti-seizure) properties and enhances lymphocyte repair of oxidative damage[84][85], identified the role of incremental folic acid levels (1 ng/mL–2 µg/mL) in stimulating in vitro human venous lymphocyte growth and reduced DNA strand breakage. Figure 7 shows the molecular structure of folate members.
Figure 7. Structures of Folate and folic acid.
It is a micronutrient essential for neurological and haematological functioning[86]. It is important in several metabolic processes because of its presence in a wide spectrum of enzymes. Dietary copper enables the synthesis of haemoglobin, neurotransmission, iron oxidation as well as peptide amidation-linked antioxidative defence[87]. Copper can scavenge or mop up free radicals by neutralizing them and in turn prevent their potential damage [88][89][90][93–95]. Dietary copper is also key to optimal skeletal functioning by enhancing collagen formation which ensures bone competence and rigidity [96]. In addition, copper functions in myelin formation which insulates nerve cells and triggers nerve impulse transmission[91]. Figure 8 indicates the molecular structure of copper.
Pantothenic acid, whose molecular structure is shown in Figure 9 is also known as Vitamin B5. This micronutritional factor is essential for the metabolism of fatty acid. Pantothenic acid has anti-stressor properties which also contribute to the production of neurotransmitters[92]. The body’s stress resistance capacity is boosted by pantothenic acid through the build-up of antibodies and it as well enhances central nervous system development [93][94].
Figure 9. Pantothenic acid structure.
3.7. Immunological and Anti-inflammatory Mechanism
Riboflavin (Vitamin B2) is thought to be implicated in the differentiation and functionality of immune cells by regulating the oxidization of fatty acid[83]. Dietary riboflavin contributes to the generation of inflammatory and immunity signalling molecules within immune cells through the mechanism of NADPH oxidase 2 priming[95]. Riboflavin exhibits anti-inflammatory capacity by suppressing the nuclear factor (NF-kB) activity[96]. Its structural molecular entity is depicted in Figure 10.
Figure 10. Riboflavin structure.
3.8. Immunological, Antioxidant and Neurological Mechanism
Pyridoxine (Vitamin B6), structurally shown in Figure 11, enhances immunity integrity via the linkage formation between chemokines and cytokines, while it enhances immune feedback towards increased antibody output[97]. Dietary pyridoxine exhibits antioxidant activity via the inhibition of erythrocytic lipid peroxidation[98] and reduces the predispositionary risk to stroke and arteriosclerosis. It is involved in synthesizing haemoglobin and neurotransmitters as well as gluconeogenesis[93][99]. Pyridoxine controls the risk of acute coronary syndrome and athero-thrombosis by modulating blood homocysteine levels[100].
Figure 11. Pyridoxine structure.
Thiamine (Vitamin B1) is centrally important in nerve functioning and energy generation from carbohydrates[93]. Thiamine modulates the neurological transmission system, improves brain functionality[101], protects the peripheral nervous system from compromise, and is involved in synthesizing myelin [102][103]. Its molecular structure is shown in Figure 12.
Figure 12. Thiamine structure.
Niacin (Vitamin B3), shown in Figure 13, is anti-inflammatory and immunohomeostatic in its activity. It inhibits the multiplicity of pro-inflammatory cytokines and the tumor necrosis factor usually effected by monocytes and macrophages [104]. Niacin deficiency in the body is symptomized by the pellagra condition which disrupts the gastrointestinal and neurological system[105][106]. A number of studies have highlighted the biological potential of niacin in anaemic, hypertensive, cardiovascular, hepatic and cancerous disease conditions [107][108][109][110][111][112].
Figure 13. Niacin structure.
The presence of phytochemicals in plants has been identified for their potential pharmacological potentials[113]. Table 3 is a compendium of dietary secondary metabolites identified in fruit components of Musa species, which reflects the broad phytomedicinal benefits derivable from their dietary intake.
mdpi/1420-3049/25/21/5036/htm#table_body_display_molecules-25-05036-t003
4. Biological Mechanism and Pharmacological Activity of Dietary Phytocompounds of Musa Species Fruits
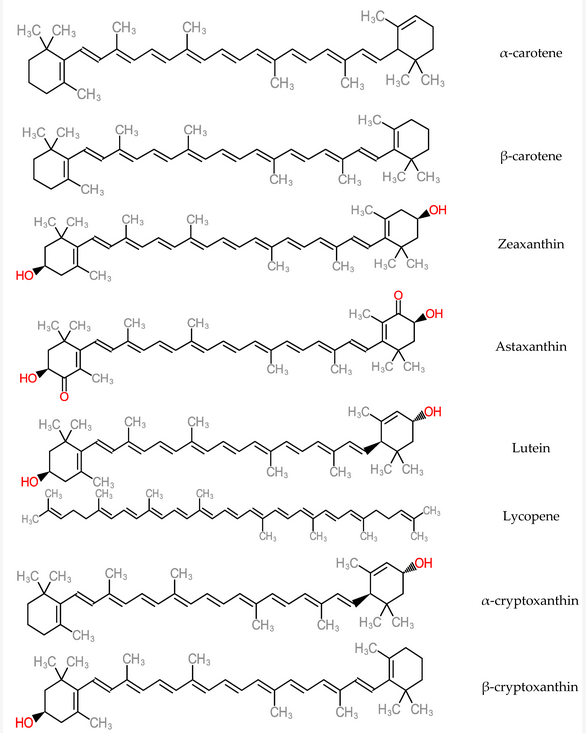
Antioxidant micronutrients, like vitamins and carotenoids, are chief contributors to the defence mechanism against reactive oxygen species (ROS) in the body[114]. Other works have reported that antioxidant vitamin and carotenoid levels were low in hepatitis and cirrhotic liver conditions[115][116]. Carotenoids are accumulated majorly in the hepatic organ and are released into blood circulation as lipoproteins. They also participate in the antioxidative defence mechanism when present in the liver and in high concentrations of free radical species. As a consequence, carotenoid physiological functions could interact with or inhibit liver dysfunctions such as acute hepatitis, hepatic steatosis, chronic hepatitis, hepatic fibrosis cirrhosis, hepatocellular carcinoma[117]. Some carotenoid members (zeaxanthin, lutein, lycopene and astaxanthin) represented in Figure 14, help in preventing the development of non-alcoholic fatty disease of the hepatic organ by mechanisms such as improvement of insulin signalling, depletion of the influx of free fatty acids into the hepatic organ[118]. Furthermore, dietary carotenoids have the capacity to reduce aging-related diseases by deploying the mechanism of reactive oxygen species production in order to inhibit cellular dysfunction and oxidative stress. These are hallmarks of antioxidative capacity [119].
α-tocopherol has been implicated in non-antioxidant mechanisms such as modulation of cell functions [120], inhibition of platelet adhesion and aggregation for blood clotting and inhibition of cytokine release[121][122][123] It also functions in phosphorylative regulation for prevention of cardiological conditions, due to its modification action on the proliferation of adhering cells and cellular oxidant production via vitamin E- specific pathways[124]. It also acts as an antioxidant in a chain-breaking mechanism against free radical propagation[124] and in the prevention of subfertility conditions (loss of spermatogenesis and poor zygote retention) on the basis on in vivo tests[125]. Tocopherols or tocotrienols are neuroprotective by inhibiting glutamate-induced death in neuronal cells[126]. Figure 15 shows the molecular structure of tocopherol members.
Catechins, shown in Figure 16, are anticancer phenolic compounds present in banana[127]. They function in mitochondrial cells in response to oxidative stress through enhanced phosphorylation, incremental production of ATP and preservation of mitochondrial membrane integrity[128]. Animal model experiments revealed corresponding effects of catechin on mitochondrial respiration and β-cell functioning[128]. Catechin has also been implicated in the upregulation of mitochondrial complexes which translates into increased ATP generation in the cell[129]. The anti-inflammatory mechanism of catechin is expressed through the modulation of transcription factors that are related to activated B cells (NF-κB) and activator protein-1 (AP-1). In vitro and animal model studies reveal dose-dependent apoptotic activity of catechin in the region of 50 µM [130][131] which further inhibits the overexpression of cyclooxygenase-2 (CoX-2)[132].
Coumarins have anti-inflammatory and antioxidant activity [133], they are anticoagulatory in the liver and are anticancerous by inhibiting the microtubule and arresting tumor cells[134]. They are also antiallergic and antiproliferative in their activity[135][136]. They exhibit strong pharmacokinetics on the basis of their easily absorbable and metabolizable nature[137]. In addition, coumarins readily function as antitumor agents and are significant because they have the capacity to counteract the side effects usually identified with the chemo and radiotherapeutic procedures[138][139][140]. Studies have identified the cytotoxic activity of coumarin compounds against leukemia cell lines (HL-60; NALM-6) and colon tumor-8[141][142] . Figure 17 depicts the molecular structure of coumarin.
Phytosterols inhibit the absorption of cholesterol by depleting the collection of metabolizable cholesterol, thereby lowering the risk of cardiovascular disease[143][144][145][146][147][148][149]. β-sitosterol has anticancer activity as seen in in vivo evidence by inhibiting the development and proliferation of breast cancer cell lines[150][151]. Furthermore, it acts as a chemopreventive actor against colon, prostate, mammary and lung cancer cells by suppressing oxidative stress[152][153][154]. Some phytosterol members are structurally depicted in Figure 18.
Figure 14. Structure of Carotenoids.
5. Dietary Incorporation of Banana and Plantain (Musa spp.)
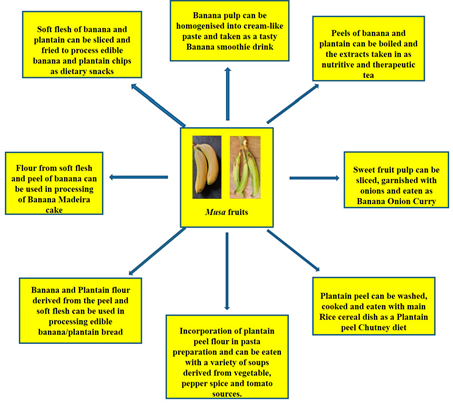
Can banana peels be eaten? This non-conventional issue has been addressed and the point is that indeed, banana peels can be eaten. Reference [155] noted that banana peels are consumed across various cultures and communities and they are simply too valuable to be perpetually disposed of. He further buttressed this point by asserting that indeed, banana peels can be eaten by human beings. Some people eat banana peels on the basis of the taste and textural modification it brings to the diet as well as the knowledge of the nutritional value present therein. Many fruits and food items may not be particularly pleasing to the eye and this, for some people is the case with banana peels as the yellow skin can be a put-off[155]. However, the many health benefits simply outweigh these sentiments. Banana peels, a major by-product of the banana processing industry are reportedly rich in fibre and nutrients, chief among are potassium, magnesium and calcium[156]. However, commercial utilization has not been maximised. Thus, the peels have notoriously become a dumping waste after consumption of the inner fruit pulp. This fate has also befallen the plantain peel, despite the huge potentials inherent.[50] has reported quite useful stores of cellulose (7.6% to 9.6%), hemicelluloses (6.4% to 9.4%) and lignin (6% to 12%) in the peels of banana. These also put banana peels in good stead as an aid in digestion processes. Creativity can be employed as the peels can be used to derive the therapeutic banana tea or perhaps a smoothie blend, just the same way the pulp has been applied as a banana smoothie and even the baked banana Madeira product (Figure 15). Essentially, the aim is to provide a more consumer-friendly outlook. Furthermore, some culinary measures have been put forward in a bid to improve the appeal of the peel products and they include: looking out for ripened banana fruits, the riper they are, the thinner and sweeter they tend to be, including thorough peel washing as a basic requirement [157]. Dieticians prescribe the intake of banana peels on the basis that the dietary fibre content ameliorates cholesterol levels in the bloodstream. Equally, plantain peels also have nutritive and dietary potentials as[158] have reported relevant protein, dietary fiber and antioxidant levels in plantain peel flour. The plantain peel flour has found utility as a substitute to wheat flour in the binding of sausage snacks [159]. [160]utilized the significant dietary fibre content in plantain peels in the derivation of cookies with high fibrous content. There is also the dietary option of incorporating plantain peel flour into pasta meal, traditionally identified with semolina flour [161]. There is also the plantain peel chutney, a local Indian recipe which is usually fried and prepared with condiments and taken in combination with a meal of rice (Figure 15). Essentially, dietary diversification potentials are inherent in banana and plantain fruits (Figure 15).
Figure 15. Dietary incorporation of Musa fruit compartments (soft flesh and peel) as contributors to improved diet diversity strategy towards alleviation to micronutrient deficiencies.
6. Production and Consumption Status of Bananas (Musa spp.)
Banana is an important subtropical and tropical fruit, which is generally cultivated in subsistence or small scale and economic or large scale across the globe as reported by[162]. According to[163], banana and plantain, members of the Musa genus are ranked fourth in export value after wheat, rice, and corn (4.5 to 5.0) billion United States Dollars (USD) per year from 1998 to 2000. According to the Food and Agricultural Organization (FAO), banana cultivation is evident in more than 130 nations and on more than 5.5 million hectares of land. A worldwide output running to 145 million tons has also been recorded [164]. In essence, with a 145-million metric ton production output worldwide (worth about £26.5 billion), Musa species are one of the globally most important staple food crops and arguably most popular international trade fruits, based on the Food and Agricultural Organization reports[165]. Reports from[166] assert that some two-thirds of the banana family cultivated and produced in west and central Africa are plantains, whose starch-rich fruits require culinary preparation for consumption, while dessert and cooking bananas make up the remaining one-third. In Africa, plantain is cultivated from Guinea to the Congo Democratic Republic and the Central African Republic. The countries with the majority production are the West African nations of Cameroon, Ghana, Nigeria and Ivory Coast (Table 4). Plantain production ranks highly in these regions (about 12327974 tonnes in 2014) among the starchy staples[166]. Plantain, as a reliable all-season staple, in developing nations faces issues of inadequate food storage, preservation and transportation technologies. In Africa, plantains and bananas supply over a quarter of carbohydrate requirements for more than 70 million people. Plantain averagely contains around 220 calories, useful potassium content and a source of dietary fiber. It is one of the major horticultural crops including the top ten most important crops in terms of food security globally and a consistent diet in rural areas and urban metropolises[167].
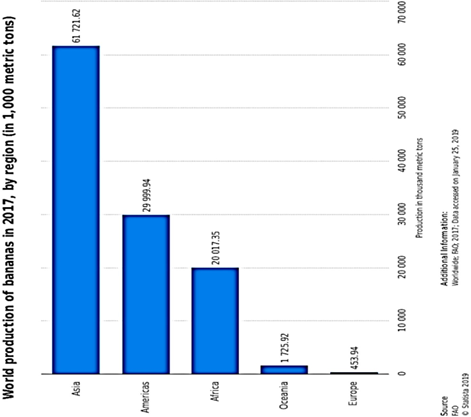
The global banana supply chain is a complex one, much dependent on several collaborations of parties. Most of the banana export stems from Ecuador, Guatemala and Costa Rica as of the year 2016. However, only about 15 to 20% of banana produced globally, end up traded in the global market. More than 1000 banana varieties are produced and consumed globally. However, Cavendish banana, accounting for 47% of global production recovers quickly from natural disaster shocks. An estimated 50 billion tonnes of Cavendish are produced yearly around the globe, and predominantly supplied to the United States of America and Europe, because they are indeed better suited to international (global) trade and possess more resilience to global travels. Precise production figures can prove a little difficult to pin down, especially because the cultivation of banana plants is majorly done by small scale farmers and trade in the informal sector. However, the available data point to the fact that between the years 2000 and 2017, the global production rate rose at about 3.2%, reaching 114 million tonnes by 2017 from 67 million tonnes in the year 2000, with Asia, the Americas and Africa being
the chief producing regions (Figure 16). Bananas and plantains make up a major daily supply of carbohydrates for about 100 million Africans[168], exemplified by Uganda, with an average annual per capita consumption of 223 kg. The banana industry has made productivity improvements from 14 to 20 tonnes per hectare from 1993 to 2017[169]. The major catalyst of increased production is the concomitant human population growth rate around the globe, surpassing seven billion people. This is, in particular, more clearly expressed in the increased consumption needs of developing countries.
Figure 16. Global production of banana showing dominant banana output in the Asian, American and African continents[164].
Table 4. The Major Global Producers of Plantain (Musa paradisiaca).
|
Rank |
Country |
Production (Tonnes) |
|
1st |
Cameroon |
4.31 million |
|
2nd |
Ghana |
3.95 million |
|
3rd |
Uganda |
3.71 million |
|
4th |
Colombia |
3.54 million |
|
5th |
Nigeria |
3.09 million |
|
6th |
Philippines |
3.07 million |
|
7th |
Peru |
2.07 million |
|
8th |
Ivory Coast |
1.59 million |
|
9th |
Myanmar |
1.11 million |
|
10th |
Democratic Republic of Congo |
1.11 million |
Source: [170].
7. Conclusions and Perspectives
This review identified and discussed a spectrum of micronutritional factors and dietary bioactive compounds in the fruit compartments (pulp and peel) of Musa species. These bioactive constituents inevitably confer a series of potential pharmacological values against oxidative damage, which helps in preventing genetic damage or DNA compromise, enhancement of immunological competence, neurological functioning and lowered risk of cardiovascular disorder. It is thus clear that banana and plantain fruit peels, as much as the flesh compartments, have a host of nutritionally and pharmacologically significant values to human nutrition, health and dietary quality. This consequently places banana and plantain peels in good stead as potential natural products and functional food options, adding credence to the need for their increased dietary utility. Summarily, given the activities described in this review, these primary and secondary metabolites exhibit properties that justify more research by the scientific community, to drive preclinical and clinical studies leading to the development of new drugs. The pharmacological insights in this review indicate the potential of Musa fruits as key elements in driving further preclinical research, in a bid to achieve natural product-based drug development from the fruits of Musa genus.
This entry is adapted from the peer-reviewed paper 10.3390/molecules25215036
References
- Batra, J.; Seth, P.K. Effect of iron deficiency on developing rat brain. Indian J. Clin. Biochem. 2002, 17, 108–114.
- Tulchisky, T.H. Micronutrient Deficiency Conditions: Global Health Issues. Public Health Rev. 2010, 32, 243–255.
- Rush, D. Nutrition and maternal mortality in the developing world. Am. J. Clin. Nutr. 2000, 1, 212–240.
- Viteri, F.E.; Gonzalez, H. Adverse outcomes of poor micronutrient status in childhood and adolescence. Nutr. Rev. 2002, 60, 77–83.
- Groce, N.; Challenger, E.; Berman-Bieler, R.; Farkas, A.; Yilmaz, N.; Schultink, W.; Clark, D.; Kerac, M. Malnutrition and disability: unexplored opportunities for collaboration. Paediatr. Int. Child. Health 2014, 34, 308–314.
- Bailey, L.B.; Stover, P.J.; McNulty, H.; Fenech, M.F.; Gregory 3rd, J.F.; Mills, J.L.; Pfeiffer, C.M.; Fazili, Z.;.Zhang, M.; Ueland, P.M. Biomarkers of Nutrition for Development- Folate Review. J. Nutr. 2015, 145, 1636–1680.
- Katona, P.; Katona-Apte, J. The interaction between nutrition and infection. Clin. Infect. Dis. 2008, 46, 1582–1588.
- Darmon, N.; Ferguson, E.L.; Briend, A. A cost constraint alone has adverse effects on food selection and nutrient density: An analysis of human diets by linear programming. J. Nutr. 2002, 132, 3764–3771.
- Chatterjee, M.; Roy, K.; Janarthan, M.; Das, S.; Chatterjee, M. Biological activity of carotenoids: its implications in cancer risk and prevention. Curr. Pharm. Biotechnol. 2012, 13, 180–190.
- Reuben, S.C.; Gopalan, A.; Petit, D.M.; Bishayee, A. Modulation of angiogenesis by dietary phytoconstituents in the prevention and intervention of breast cancer. Mol. Nutr. Food Res. 2012, 56, 14–29.
- Nechuta, S.; Lu, W.; Zheng, Y.; Cai, H.; Bao, P.; Gu, K.; Zheng, W.; Shu, X.O. Comorbidities and breast cancer survival: a report from the Shanghai breast cancer survival study. Breast Cancer Res. Treat. 2013, 139, 227–235.
- Fink, B.N.; Steck, S.E.; Wolff, M.S.; Britton, J.A.; Kabat, G.C.; Schroeder, J.C.; Teitelbaum, S.L.; Neugut, A.I.; Gammon, M.D. Dietary flavonoid intake and breast cancer risk among women on Long Island. Am. J. Epidemiol. 2007, 165, 514–523.
- Cutler, G.J.; Nettleton, J.A.; Ross, J.A.; Harnack, L.S.; Jacobs Jr. D.R.; Scrafford, C.G.; Barraj, L.M.; Mink, P.J.; Robien, K. Dietary flavonoid intake and risk of cancer in post-menopausal women: The Iowa Women's Health Study. Int. J. Cancer. 2008, 123, 664–671.
- Kresty, L.A.; Morse, M.A.; Morgan, C.; Carlton, P.S.; Lu, J.; Gupta, A.; Blackwood, M.; Stoner, GD. Chemoprevention of esophageal tumorigenesis by dietary administration of lyophilized black raspberries. Cancer Res. 2001, 61, 6112–6119.
- Hagiwara, A.; Miyashita, K.; Nakanishi, T.; Sano, M.; Tamano, S.; Kadota, T.; Koda, T.; Nakamura, M.; Imaida, K.; Ito, N. et al. Pronounced inhibition by a natural anthocyanin, purple corn color, of 2-amino-1-methyl-6-phenylimidazo [4,5-b] pyridine (PhIP)-associated colorectal carcinogenesis in male F344 rats pretreated with 1, 2-dimethylhydrazine. Cancer Lett. 2001, 171, 17–25.
- Schaefer, S.; Baum, M.; Eisenbrand, G.; Janzowski, C. Modulation of oxidative cell damage by reconstituted mixtures of phenolic apple juice extracts in human colon cell lines. Mol. Nutr. Food Res. 2006, 50, 413–417.
- Rolls, B.J.; Ello-Martin, J.A.; Tohill, B.C. What can intervention studies tell us about the relationship between fruit and vegetable consumption and weight management? Nutr.Rev. 2004, 62, 1–17.
- Tohill, B.C.; Seymour, J.; Serdula, M.; Kettel-Khan, L.; Rolls, B.J. What Epidemiologic Studies Tell Us about the Relationship between Fruit and Vegetable Consumption and Body Weight? Nutr. Rev. 2004, 62, 365–374.
- Rautiainen, S.; Wang, L.; Lee, I.; Manson, J.E.; Buring, J.E.; Sesso, H.D. Higher intake of fruit, but not vegetables or fiber, at baseline is associated with lower risk of becoming overweight or obese in middle-aged and older women of normal BMI at baseline1-3. J. Nutr. 2015, 145, 960–968.
- Lock, K.; Pomerleau, J.; Causer, L.; Altmann, D.R.; McKee, M. The global burden of disease attributable to low consumption of fruit and vegetables: implications for the global strategy on diet. Bull.World Health Organ. 2005, 83, 100–108.
- Boeing, H.; Bechthold, A.; Bub, A.; Ellinger, S.; Haller, D.; Kroke, A.; Leschik-Bonnet, E.; Muller, M.J.; Oberritter, H.; Schulze, M. et al. Critical review: vegetables and fruit in the prevention of chronic diseases. Eur. J. Nutr. 2012, 51, 637–663.
- Yu, N.; Su, X.; Wang, Z.; Dai, B.; Kang, J. Association of Dietary Vitamin A and β-carotene intake with the risk of lung cancer: A meta-analysis of 19 Publications. Nutrients 2015, 7, 9309–9932.
- Du, H.; Li, L.; Bennett, D.; Guo, Y.; Key, T.J.; Bian, Z.; Sherliker, P.; Gao, H.; Chen, Y.; Yang, L. et al. Fresh fruit consumption and major cardiovascular disease in China. N. Engl. J. Med. 2016, 374, 1332–1343.
- Zhai, H.; Wang, Y.; Jiang, W. Fruit and vegetable intake and the risk of chronic obstructive pulmonary disease: a dose-response meta-analysis of observational studies. B.M.R.I. 2020, 2020, 1–12.
- Constantine, D.; Rossel, G. The Musaceae: An annotated list of the species of Ensete, Musa and Musella, 2001.
- Boning, C.R. Florida’s Best Fruiting Plants, 1st ed.; Pineapple Press Inc.: Florida, USA, 2006; pp. 1-232.
- Perriera, X.; De Langheb, E.; Donohuec, M.; Lentferd, C.; Vrydaghse, L.; Bakrya, F.; Carreel, F.; Hippolyte, I.; Horry, J.-P.; Jenny, C. et al. Multidisciplinary perspectives on banana (Musa spp.) domestication. Proc. Natl. Acad. Sci. U.S.A 2011, 108, 11311–11318.
- Sampath Kumar, K.P.; Bhowmik, D.; Duraivel, S.; Umadevi, M. Traditional and Medicinal Uses of Banana. J. Pharm. . Phytochem. 2012, 1, 51–63.
- Khawas, P.; Deka, S.C. Comparative nutritional, functional, morphological and diffractogram study on culinary banana (Musa ABB) peel at various stages of development. Int. J. Food Prop. 2016, 19, 2832–2853.
- Hill, A. Plantains vs Bananas: What's The Difference? In: Health line Newsletter, Nutrition, 2018; pp. 1-12.
- Sulaiman, S.F.; Yusoff, N.A.M.; Eldeen, I.M.; Seow, E.M.; Sajak, A.A.B.; Supriatno, Ooi, K.L. Correlation total phenolic and mineral contents with antioxidant activity of eight malaysian bananas (Musa sp.). J. Food Compos. Anal. 2011, 24, 1–10.
- Aurore, G.; Parfait, B.; Fahrasmane, L. Bananas, raw materials for making processed food products. Trends Food Sci. Technol. 2009, 20, 78–91.
- Bennett, R.N.; Shiga, T.M.; Hassimotto, N.M.; Rosa, E.A.; Lajolo, F.M.; Cordenunsi, B.R. Phenolics and antioxidant properties of fruit pulp and cell wall fractions of post-harvest banana (Musa acuminata Juss.) cultivars. J. Agric. Food Chem. 2010, 58, 7991–8003.
- Menezes, E.W.; Tadini, C.C.; Tribess, T.B.; Zuleta, A.; Binaghi, J.; Pak, N.; Vera, G.; Dan, M.C.T.; Bertolini, A.C.; Cordenunsi, B.R. et al. Chemical composition and nutritional value of unripe banana flour (Musa acuminata var. Nanicão). Plant. Foods Hum. Nutr. 2011, 66, 231–237.
- Sarawong, C.; Schoenlechner, R.; Sekiguchi, K.; Berghofer, E.; Ng, P.K.W. Effect of extrusion cooking on the physicochemical properties, resistant starch, phenolic content and antioxidant capacities of green banana flour. Food Chem. 2014, 143, 33–39.
- Faller, A.L.K.; Fialho, E. Polyphenol content and antioxidant capacity in organic and conventional plant foods. J. Food Compos. Anal. 2010, 23, 561–568.
- Fu, L.; Xu, B.T.; Xu, X.R.; Gan, R.Y.; Zhang, Y.; Xia, E.Q.; Li, H.B. Antioxidant capacities and total phenolic contents of 62 fruits. Food Chem. 2011, 129, 345–350.
- Aina, D.A.; Jonathan, S.G.; Olawuyi, O.J.; Ojelabi, D.O.; Durowoju, B.M. Antioxidant, antimicrobial and phytochemical properties of alcoholic extracts of Cantharellus cibarius: a Nigerian mushroom. N. Y. Sci. J. 2012, 5, 114–120.
- Oyeyinka, B.O.; Afolayan, A.J. Comparative evaluation of the nutritive, mineral and antinutritive compositions of Musa sinensis L. (Banana) and Musa paradisiaca L. (Plantain) fruit compartments. Plants 2019, 8, 1–14.
- Feumba, D.R.; Ashwini, R.P.; Ragu, S.M. Chemical composition of some selected fruit peels. E.J.F.S.T. 2016, 4, 12–21.
- Mahon, B.D.; Gordon, S.A.; Cruz, J.; Cosman, F.; Cantorna, M.T. Cytokine profile in patients with multiple Sclerosis following Vitamin D supplementation. J. Neuroimmunol. 2003, 134, 128–132.
- Schleithoff, S.S.; Zittermann, A.; Tenderich, G.; Berthold, H.K.; Stehle, P.; Koerfer, R. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: A double-blind, randomized, placebo-controlled trial. Am.J. Clin. Nutr. 2006, 83, 754–759.
- Adegbaju, O.D.; Otunola, G.A.; Afolayan, A.J. Potential of Celosia species in alleviating micronutrient deficiencies and prevention of diet-related chronic diseases: a review. Aims Agric. Food 2019, 4, 458–484.
- Christian, P.; Stewart, C.P. Maternal micronutrient deficiency, fetal development and the risk of chronic disease. J. Nutr. 2010, 140, 437–445.
- Jomova, K.; Valko, M. Advances in metal-induced oxidative stress and human disease. Toxicology 2011, 283, 65–87.
- Adeyemi, O.S.; Oladiji, A.T. Compositional changes in banana (Musa ssp.) fruits during ripening. Afr. J. Biotechnol. 2009, 8, 858–859.
- Selema, M.D.; Farago, M.E. Trace element concentrations in the fruit peels and trunks of Musa paradisiaca. Phytochemistry 1996, 42, 1523–1525.
- Davey, M.W.; Van den Bergh, I.; Markham, R.; Swennen, R.; Keulemans, J. Genetic variability in Musa fruit provitamin A carotenoids, lutein and mineral micronutrient contents. Food Chem. 2009, 115, 806–813.
- Offem, J.O.; Njoku, P.C. Mineral distribution in the fruits of the plantain plant (Musa paradisiaca) in relation to mode and degree of maturation. Food Chem. 1993, 48, 63–68.
- Anderson, D.L.; Cunningham, W.C.; Lindstrom, T.R. Concentrations and intakes of H, B, S, K, Na, Cl and NaCl in foods. J. Food Comp. Anal. 1994, 7, 59–82.
- Emaga, T.H.; Ronkart, S.N.; Robert, C.; Wathelet, B.; Paquot, M. Characterization of pectins extracted from banana peels (Musa AAA) under different conditions using an experimental design. Food Chem. 2008, 108, 463–471.
- Stefanidou, M.; Maravelias, C.; Dona, A.; Spiliopoulou, C. Zinc: A multipurpose trace element. Arch. Toxicol. 2006, 80, 1–9.
- Chasapis, C.T.; Loutsidou, A.C.; Spiliopoulou, C.A.; Stefanidou, M.E. Zinc and Human Health: An Update. Arch. Toxicol. 2012, 86, 521–534.
- Olechnowicz, J.; Tinkov, A.; Skalny, A.; Suliburska, J. Zinc status is associated with inflammation, oxidative stress, lipid and glucose metabolism. J. Physiol. Sci. 2018, 68, 19–31.
- Jarosz, M.; Szkaradek, N.; Marona, H.; Nowak, G.; Młyniec, K.; Librowsk, T.Evaluation of anti-inflammatory and ulcerogenic potentialof zinc-ibuprofen and zinc-naproxen complexes in rats. Inflammopharmacol. 2017, 25, 653–663.
- Prasad, S.N. Mitigation of acrylamide-induced behavioral deficits, oxidative impairments and neurotoxicity by oral supplements of geraniol (a monoterpene) in a rat mode. Chem.Biol. Interact. 2014, 223, 27–37.
- Cortese, S.; Angriman, M.; Maffeis, C.; Isnard, P.; Konofal, E.; Lecendreux, M.; Purper-Ouakil, D.; Vincenzi, B.; Bernardina, B.D.; Mouren, M.C. Attention - Deficit/Hyperactivity disorder (ADHD) and Obesity: A Systematic Review of the Literature. Crit. Rev. Food Sci. Nutr. 2008, 48, 524–537.
- Csermely, P.; Somogyi, J. Zinc as a Possible Mediator of Signal Transduction in T-Lymphocytes. Acta Physiol. Hung. 1989, 74, 195–199.
- Haase, H.; Rink, L. Functional Significance of Zinc-Related Signaling Pathways in immune cells. Annu. Rev. Nutr. 2009, 29, 133–152.
- Fraker, P.J.; Gershwin, M.E.; Good, R.A.; Prasad, A. Interrelationships between zinc and immune function. Fed. Proc. 1986, 45, 1474–1479.
- Hojyo, S.; Fukada, T. Zinc transporters and signaling in physiology and pathogenesis. Arch. Biochem. Biophys. 2016, 611, 43–50.
- Mott, D.D.; Dingledine, R. Unraveling the role of zinc in memory. P.N.A.S. 2011, 108, 3103–3104.
- Besser, L.; Chorin, E.; Sekler, I.; Silverman, W.F.; Atkin, S.; Russell, J.T.; Hershfinker, M. Synaptically released zinc triggers metabotropic signaling via a zinc-sensing receptor in the Hippocampus. J. Neurosci. 2009, 29, 2890–2901.
- Sindreu, C.; Palmiter, R.; Storm, D. Zinc transporter ZnT-3 regulates presynaptic Erk1/2 signaling and hippocampus-dependent memory. Proc. Natl. Acad. Sci. 2011, 108, 3366–3370.
- Penland, J.G. The importance of Boron nutrition for brain and psychological functions. Biol. Trace Elem. Res. 1998, 66, 299–317.
- Coban, F.K.; Liman, R.; Cigerci, I.H.; Ince, S.; Hazman, O.; Bozkurt, M.F. The antioxidant effect of boron on oxidative stress and DNA damage in diabetic rats. Fresen. Env. . Bull. 2015, 24, 4059–4066.
- Cao, J.; Jiang, L.; Zhang, X.; Yao, X.; Geng, C.; Xue, X.; Zhong, L.F. Boric acid inhibits LPS-induced TNF-alpha formation through a thiol-dependent mechanism in THP-1 cells. J. Trace Elem. Med. Biol. 2008, 22, 189–195.
- Zafar, H.; Ali, S. Boron inhibits the proliferating cell Nuclear Antigen Index, molybdenum containing proteins and ameliorates oxidative stress in hepatocellular carcinoma. Arch. Biochem. Biophys. 2013, 529, 66–74.
- Khaliq, H.; Juming, Z.; Ke-Mei, P. The Physiological Role of Boron on Health. Biol. Trace Elem. Res. 2018, 186, 31–51.
- Demirdogen, R.E. Relationship among blood boron level, diabetes mellitus, lipid metabolism, bone metabolism and obesity: Can boron be an efficient indicator for metabolic diseases? Health Sci. J. 2020, 14, 1–11.
- Barranco, W.T.; Eckhert, C.D. Boric acid inhibits prostate cancer cell proliferation. Cancer Lett. 2004, 216, 21–29.
- Barranco, W.T.; Eckhert, C.D. Cellular changes in boric acid-treated DU-145 prostate cancer cells. Br. J. Cancer 2006, 94, 884–890.
- Korkmaz, M.; Uzgo, E.; Bakirdere, S.; Aydin, F.; Ataman, Y. Effects of dietary boron on cervical cytopathology and on micronucleus frequency in exfoliated buccal cells. Env. . Toxicol. 2007, 22, 17–25.
- Gupta, G.P. Role of Iron (Fe) in Body. Iosr J.A.C. 2014, 7, 38–46.
- Scholl, T.O.; Stein, T.P.; Smith, W.K. Leptin and maternal growth during adolescent pregnancy. Am. J. Clin. Nutr. 2000, 72, 1542–1547.
- West Jr., K.P.; Katz, J.; Khatry, S.K.; LeClerq, S.C.; Pradhan, E.K.; Shrestha, S.R.; Connor, P.B.; Dali, S.M.; Christian, P.; Pokhrel, R.P. et al. Double blind, cluster randomised trial of low dose supplementation with vitamin A or beta-carotene on mortality related to pregnancy in Nepal, The NNIPS-2 study group. Br. Med. J. 1999, 318, 570–575.
- Finley, J.W.; Davis, C.D. Manganese absorption and retention in Rats is affected by the type of dietary fat. Biol. Trace Elem. Res. 2001, 82, 143–158.
- Merrell, P.H. The Importance of Minerals in the Long Term Health of Humans. Jost Chemical Company, 2017, pp. 1-21.
- Stover, P.J. Physiology of Folate and Vitamin B12in Health and Disease. Nutr. Rev. 2004, 62, 3–12.
- Huskinsson, E.; Maggini, S.; Ruf, M. The influence of micronutrients on cognitive function and performance. J. Int. Med. Res. 2007, 35, 1–19.
- Duthie, S.J. Folic Acid Deficiency and Cancer: Mechanisms of DNA Instability. Br.Med. Bull. 1999, 55, 578–592.
- Courtemanche, C.; Elson-Schwab, I.; Mashiyama, S.T.; Kerry, N.; Ames, B.N. Folate deficiency inhibits the proliferation of primary human CD8+ T lymphocytes in vitro. J. Immunol. 2004, 173, 3186–3192.
- Yoshii, K.; Hosomi, K.; Sawane, K.; Kunisawa. Metabolism of dietary and microbial vitamin B family in the regulation of host immunity. J. Front. Nutr. 2019, 6, 1–12.
- Reynolds, E.H.; Preece, J.; Johnson, A.L. Folate metabolism in epileptic and psychiatric patients. J. Neurol. Neurosurg. Psychiatry 1971, 34, 726–732.
- Duthie, S.J.; Hawdon, A. DNA instability (strand breakage, uracil misincorporation and defective repair) is increased by folic acid depletion in human lymphocytes in vitro. F.A.S.E.B. J. 1998, 12, 1491–1497.
- Al-Fartusie, F.S.; Mohssan, S.N. Essential Trace Elements and Their Vital Roles in human body. Indian J. Adv. Chem. Sci. 2017, 5, 127–136.
- Myint, Z.W.; Oo, T.H.; Thein, K.Z.; Tun, A.M.; Saeed, H. Copper Deficiency Anemia: Review Article. Ann. Hematol. 2018, 97, 1527–1534.
- Bonham, M.; O'Connor, J.M.; Hannigan, B.M.; Strain, J.J. The immune system as a physiological indicator of marginal copper status? Br. J. Nutr. 2002, 87, 393–403.
- Davis, C.D. Low dietary copper increases fecal free radical production, fecal water alkaline phosphatase activity and cytotoxicity in healthy men. J. Nutr. 2003, 133, 522–527.
- Araya, M.; Pizarro, F.; Olivares, M.; Arredondo, M.; Gonzalez, M.; Mendez, M. Understanding copper homeostasis in humans and copper effects on health. Biol. Res. 2006, 39, 183–187
- Copper Development Association. Copper: Essential for Human Health, Copper Alliance, 2018.
- Kaur, A. Biological functions of Vitamin B complex and effects on human health in both excess and deficiency
- Kaur, A. Biological functions of Vitamin B complex and effects on human health in both excess and deficiency levels. PharmaTutor 2015, 3, 40–47.
- Rucker, R.; Bauerly, K. Pantothenic Acid. Handbook of Vitamins, 5th ed. (eds: Zempleni, J., Suttie, J., Jesse, F. Gregory III, Patrick J. Stover), Chapter 8, CRC Press, Boca Raton, FL, 2013, pp. 325–349.
- Schramm, M.; Wiegmann, K.; Schramm, S.; Gluschko, A.; Herb, M.; Utermohlen, O.; Kr ̈onke, M. Riboflavin (vitamin B2) deficiency impairs NADPH oxidase 2 (Nox2) priming and defense against Listeria monocytogenes. Eur. J. Immunol. 2014, 44, 728–741.
- Marashly, E.T.; Bohlega, S.A. Riboflavin has neuroprotective potential: focus on parkinson’s disease and migraine. Front. Neurol. 2017, 8, 1–12.
- Kunisawa, J.; Kiyono, H. Vitamin-mediated regulation of intestinal immunity. Front. Immunol. 2013, 4, 1–6.
- Velásquez, M.; Méndez, D.; Moneriz, C. Pyridoxine decreases oxidative stress on human erythrocyte membrane protein in vitro. TOBIOCJ. 2019, 13, 37–44.
- Bradford, A. Vitamin B6: Sources and Benefits. LiveScience; https://www.livescience.com/51920-vitamin-b6.html; 2015, pp. 1-13.
- Vermeulen, E.G.; Stehouwer, C.D.; Twisk, J.W.; van den Berg, M.; de Jong, S.C.; Mackaay, A.J. Campen, C.V.; Visser, F.C.; Jakobs, C.; Bulterijs, E.J. Effect of Homocysteine-Lowering Treatment with Folic Acid plus Vitamin B6 on progression of subclinical atherosclerosis: A randomised, placebo-controlled Trial. Lancet. 2000, 355, 517–522.
- Hirsch, J.A.; Parrott, J. New considerations on the neuromodulatory role of Thiamine. Pharmacology 2012, 89, 111–116.
- Singleton, C.K.; Martin, P.R. Molecular mechanisms of Thiamine Utilization. Curr. Mol. Med. 2001, 1, 197–207.
- Calderón-Ospina, C.A.; Nava-Mesa, M.O. B vitamins in the nervous system: Current knowledge of the biochemical modes of action and synergies of thiamine, pyridoxine, and cobalamin. Cns Neurosci. 2020, 26, 5–13.
- Lipszyc, P.S.; Cremaschi, G.A.; Zubilete, M.Z.; Bertolino, M.L.A.; Capani, F.; Genaro, A.M.; Wald, M.R. Niacin modulates pro-inflammatory cytokine secretion: A potential mechanism involved in its anti-atherosclerotic effect. Open Cardiovasc. Med. J. 2013, 7, 90–98.
- Hegyi, J.; Schwartz, R.A.; Hegyi, V. Pellagra: Dermatitis, dementia, and diarrhea. Int. J. Derm. . 2004, 43, 1–5.
- Amanullah, S.; Seeber, C. Niacin deficiency resulting in neuropsychiatric symptoms: A case study and review of literature. Clin. Neuropsychiatry 2010, 7, 10–14.
- Arun, P.; Padmakumaran Nair, K.G.; Manojkumar, V.; Deepadevi, K.V.; Lakshmi, L.R.; Kurup, P.A. Decreased hemolysis and lipid peroxidation in blood during storage in the presence of nicotinic acid. Vox. Sang. 1999, 76, 220–225.
- Boyonoski, A.C.; Gallacher, L.M.; ApSimon, M.M.; Jacobs, R.M.; Shah, G.M.; Poirier, G.G.; Kirkland, J.B. Niacin deficiency in rats increases the severity of Ethylnitrosourea-induced Anemia and Leukopenia. J. Nutr. 2000, 130, 1102–1107.
- Yuvaraj, S.; Premkumar, V.G.; Vijayasarathy, K.; Gangadaran, S.G.D.; Sachdanandam, P. Augmented antioxidant status in tamoxifen treated post-menopausal women with breast cancer on co-administration with coenzyme q10, niacin and riboflavin. Cancer Chemother. Pharm. . 2008, 61, 933–941.
- Cho, K.; Kim, H.; Rodriguez-Iturbe, B.; Vaziri, N.D. Niacin ameliorates oxidative stress, inflammation, proteinuria, and hypertension in rats with chronic renal failure. Am. J. Physiol. Ren. Physiol. 2009, 297, 106–113.
- Ganji, S.H.; Kashyap, M.L.; Kamanna, V.S. Niacin inhibits fat accumulation and oxidative stress in human hepatocytes and regresses hepatic steatosis in experimental rat model. J. Clin. Lipidol. 2014, 8, 349–350.
- Ganji, S.H.; Kashyap, M.L.; Kamanna, V.S. Niacin inhibits fat accumulation, oxidative stress and inflammatory cytokine IL-8 in cultured hepatocytes: Impact on non-alcoholic fatty liver disease. Metabolism. 2015, 64, 982–990.
- Wall, M. Ascorbic acid, vitamin A, and mineral composition of banana (Musa sp.) and papaya (Carica papaya) cultivars grown in Hawaii. J. Food Comp. Anal. 2006, 19, 434–445.
- Stanner, S.A.; Hughes, J.; Kelly, C.N.; Buttriss, J. A review of the epidemiological evidence for the ‘antioxidant hypothesis’. Public Health Nutr. 2004, 7, 407–422.
- Jain, S.K.; Pemberton, P.W.; Raymond, A.S.; McMahon, F.T.; Burrows, P.C.; Aboutwerat, A.; Warnes, T.W. Oxidative stress in chronic hepatitis C: not just a feature of late stage disease. J. Hepatol. 2002, 36, 805–811.
- Van de Casteele, M.; Zaman, Z.; Zeegers, M.; Servaes, R.; Fevery, J.; Nevens, F. Blood antioxidant levels in patient with alcoholic liver disease correlate with the degree of liver impairment and are not specific to alcoholic liver injury itself. Aliment. Pharm. . . 2002, 16, 985–992.
- Sugiura, M. Bioactive food as dietary interventions for liver and gastrointestinal disease. Chapter 27- Carotenoids, In: Liver Diseases Prevention, 2013, pp. 421–436.
- Murillo, A.G.; Di Marco, D.M.; Fernandez, M.L. The potential of non-provitamin A carotenoids for the prevention and treatment of non-alcoholic fatty liver disease. Biology 2016, 5, 1–15.
- Tan, B.L.; Norhaizan, M.E. Curcumin Combination Chemotherapy: The implication and efficacy in cancer. Molecules 2019, 24, 1–21.
- Devaraj, S.; Jialal, I. The effects of alpha-tocopherol on critical cells in atherogenesis. Curr. Opin. Lipidol. 1998, 9, 11–15.
- Cecchini, T.; Ciaroni, S.; Ferri, P.; Ambrogini, P.; Cuppini, R.; Santi, S.; Grande, P.D. Alpha-tocopherol, an exogenous factor of adult hippocampal neurogenesis regulation. J. Neurosci. Res. 2003, 73, 447–455.
- Rimbach, G.; Minihane, A.M.; Majewicz, J.; Fischer, A.; Pallauf, J.; Virgli, F.; Weinberg, P.D. Regulation of cell signalling by Vitamin E. Proc. Nutr. Soc. 2002, 61, 415–425.
- van Aalst, J.A.; Burmeister, W.; Fox, P.L.; Graham, L.M. α-tocopherol preserves endothelial cell migration in the presence of cell-oxidized low-density lipoprotein by inhibiting changes in cell membrane fluidity. Eur. J. Vasc. Surg. 2004, 39, 229–237.
- Brigelius-Flohé, R.; Traber, M.G. Vitamin E: function and metabolism. The FASEB Journal, 1999, 13, 1145–1155.
- Evans, H.M.; Bishop, K.S. On the existence of a hitherto unrecognized dietary factor essential for reproduction. Science 1922, 56, 650–661.
- Sen, C.K.; Khanna, S.; Roy, S.; Packer, L. Molecular basis of vitamin E action: Tocotrienols potently inhibit glutamate-induced pp60c-scr kinase activation of HT4 neuronal cells. J. Biol. Chem. 2000, 275, 13049–13055.
- Williamson, G.; Manach, C. Bioavailability and bioefficacy of polyphenols in humans II: Review of 93 intervention studies. Am. J. Clin. Nutr. 2005, 81, 243–255.
- Bernatoniene, J.; Kopustinskiene, D.M. The role of catechins in cellular responses to oxidative stress. Molecules 2018, 23, 1–11.
- Rowley, T.J.; Bitner, B.F.; Ray, J.D.; Lathen, D.R.; Smithson, A.T.; Dallon, B.W.; Plowman, C.J.; Bikman, B.T.; Hansen, J.M.; Dorenkott, M.R.; et al. Monomeric cocoa catechins enhance β-cell function by increasing mitochondrial respiration. J. Nutr. Biochem. 2017, 49, 30–41.
- Braicu, C.; Gherman, C.D.; Irimie, A.; Berindan-Neagoe, I. Epigallocatechin-3-Gallate (EGCG) inhibits cell proliferation and migratory behaviour of triple negative breast cancer cells. J. Nanosci. Nanotechnol. 2013, 13, 632–637.
- Weinreb, O.; Amit, T.; Mandel, S.; Youdim, M.B.H. Neuroprotective molecular mechanisms of (-)-epigallocatechin-3-gallate: a reflective outcome of its antioxidant, iron chelating and neuritogenic properties. Genes Nutr. 2009, 4, 283–496.
- Shirakami, Y.; Sakai, H.; Kochi, T.; Seishima, M.; Shimizu, M. Catechins and Its Role in Chronic Diseases. Adv. Exp. Med. Biol. 2016, 929, 67-90.
- Hadjipavlou-Litina, D.; Kontogiorgis, C.; Pontiki, E.; Dakanali, M.; Akoumianaki, A.; Katerinopoulos, H.E. Anti-inflammatory and antioxidant activity of coumarins designed as potential fluorescent zinc sensors. J. Enzym. Inhib. Med. Chem. 2007, 22, 287–292.
- Kim, S.; Kim, N.H.; Park, Y.S.; Kim, H.; Lee, S.; Wang, Q.; Kim, Y.K. 7-diethylamino-3(2'-benzoxazolyl)-coumarin is a novel microtubule inhibitor with antimitotic activity in multidrug resistant cancer cells. Biochem. Pharm. . 2009, 77, 1773–1779.
- Sandeep, G.; Sri Ranganath, Y.; Bhasker, S.; Rajkumar, N. Synthesis and Biological Screening of Some Novel Coumarin Derivatives. Asian J. Res. Chem. 2009, 2, 46–48.
- Ramesh, B.; Sumana, T. Synthesis and anti-inflammatory activity of Pyrazolines. E-J.Chem. 2010, 7, 514–516.
- Pelkonen, O.; Raunio, H.; Rautio, A.; Pasanen, M.; Lang, M.A. The metabolism of coumarin. In: O’Kennedy, R. & Thornes, R.D., Coumarins: Biology, Applications and Mode of Action, New York, John Wiley, 1, 1997, pp. 67–92.
- Loprinzi, C.L.; Kugler, J.W.; Sloan, J.A.; Rooke, T.W.; Quella, S.K.; Novotny, P.; Mowat, R.B.; Michalak, J.C.; Stella, P.J.; Levitt, R. et al. Lack of effect of coumarin in women with lymphedema after treatment for breast cancer. N. Engl. J. Med. 1999, 340, 346–350.
- Finn, G.; Kenealy, E.; Creaven, B.; Egan, D. Coumarinyl aldehyde as a Michael acceptor type of calorimetric and fluorescent probe for cyanide in water. Cancer Letts, 2002, 18, 54–61.
- Rohini, K.; Srikumar, P.S. Therapeutic role of coumarins and coumarin-related compounds. J. . Catal. 2014, 5, 1–3.
- Groutas, W.C.; Abrams, W.R.; Carroll, R.T.; Moi, M.K.; Miller, K.E.; Margolis, M.T. Specific inhibition of human leukocyte elastase by substituted alpha-pyrones, Experientia. 1984, 40, 361–362.
- Budzisz, E.; Brzezinska, E.; Krajewska, U.; Rozalski, M. Cytotoxic effects, alkylating properties and molecular modelling of coumarin derivatives and their phosphonic analogues. Eur. J. Med. Chem. 2003, 38, 597–603.
- Ostlund, R.E. Phytosterols and cholesterol metabolism. Curr. Opin. Lipidol. 2004, 15, 37–41.
- Cohn, J.S.; Kamili, A.; Wat, E.; Chung, R.W.S.; Tanday, S. Dietary phospholipids and intestinal cholesterol absorption. Nutrients 2010, 2, 116–127.
- Lin, X.; Racette, S.B.; Lefevre, M.; Spearie, C.A.; Most, M.; Ma, L.; Ostlund, Jr., R.E. The effects of phytosterols present in natural food matrices on cholesterol metabolism and LDL-cholesterol: a controlled feeding trial. Eur. J. Clin. Nutr. 2010, 64, 1481–1487.
- Lin, X.; Ma, L.; Racette, S.B.; Spearie, C.L.A.; Ostlund Jr., R.E. Phytosterol glycosides reduce cholesterol absorption in humans. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, 931–935.
- Gylling, H.; Plat, J.; Turley, S.; Ginsberg, H.N.; Ellegård, L.; Jessup, W.; et al. Plant sterols and plant stanols in the management of dyslipidaemia and prevention of cardiovascular disease. Atherosclerosis 2014, 232, 346–360.
- Gylling, H.; Simonen, P. Phytosterols, phytostanols and lipoprotein metabolism. Nutrients 2015, 7, 7965–7977.
- Dumolt, H.; Rideout, T.C. The lipid-lowering effects and associated mechanisms of dietary phytosterol supplementation. Curr. Pharm. Des. 2017, 23, 5077–5085.
- Ju, L.M.; Clausen, K.F.; Allred, A.L.; Almada, W.G. Helferich, β-sitosterol, β-sitosterol glucoside, and a mixture of β-sitosterol and β-sitosterol glucoside modulate the growth of estrogen-responsive breast cancer cells in vitro and in ovariectomized athymic Mice. Nutr. Cancer 2004, 134, 1145–1151.
- Awad, A.B.; Chinnam, M.; Fink, C.S.; Bradford, P. Beta-Sitosterol activates Fas signaling in human breast cancer cells. Phytomed. 2007, 14, 747–754.
- Awad, A.B.; Downie, A.; Fink, C.S.; et al. Dietary phytosterol inhibits the growth and metastasis of MDA-MB-231 human breast cancer cells grown in SCID mice. Anticancer Res., 2000, 20, 821–824.
- Awad, M.M.; Ellemor, D.M.; Boyd, R.L.; Emmins, J.J.; Rood, J.I. Synergistic effects of alpha-toxin and perfringolysin O in Clostridium perfringens-mediated gas gangrene. Infect. Immun. 2001, 69, 7904–7910.
- Awad, A.B.; Barta, S.L.; Fink, C.S.; Bradford, P.G. beta-Sitosterol enhances Tamoxifen effectiveness on breast cancer cells by affecting ceramide metabolism. Mol. Nutr. Food Res. 2008, 52, 419–426.
- Pramod Kerkar. Can You Eat Banana Peel? https://www.epainassist.com/articles/can-you-eat-banana-peel, 2019, pp. 1–7.
- Megha Karthikeyan, Suma Divakar. Standardization of banana peel based sauce. Int. J. Food Sci. Nutr. 2018, 3, 95–98.
- Melissa Breyer. How to eat banana peels. Tree Hugger: Living/Green Food, 2016, pp. 1–3.
- Agama-Acevedo, E.; Sañudo-Barajas, J.A.; Vélez De La Rocha, R.; González Aguilar, G.A.; Bello-Peréz, L.A. Potential of plantain peels flour (Musa paradisiaca L.) as a source of dietary fiber and antioxidant compound. J. Food, 2016, 14, 117–123.
- Chasoy, G.R.; Cock, L.S. Effect of plantain (Musa paradisiaca L. cv. Dominico Harton) peel flour as binder in frankfurter-type sausage. Acta Agronomica, 2017, 66, 305–310.
- Arun, M.; Satish, S.; Anima, P. Experimental wound healing aspects of Jasminum grandiflorum L.: A pre-clinical study. Aliment. Pharm. 2015, 12, 135–142.
- Aguilar, J.M.C.; Baria, A.R.E.; Cruz, C.O. Incorporation of Saba (Musa paradisiaca L.) banana peel in fresh pasta. Antorcha, 2016, 3, 1–21.
- Banerjee, S.; Halder, B.; Barman, N.R.; Ghosh, A.K. An overview on different variety of Musa species: Importance and its enormous pharmacological action. J. Pharm.Herbal Formulations, 2010, 1, 2–11.
- Marín, D.H.; Romero, R.A.; Guzmán, M.; Sutton, T.B. Black Sigatoka: An increasing threat to banana cultivation. A.P.S., 2003, 87, 208–222.
- FAO. The State of Food Security and Nutrition in the World: Building Resilience For Peace and Food Security. Food and Agriculture Organization of the United Nations Rome, 2017, pp. 1–27.
- FAO. The changing face of global banana trade, Rome, 2014.
- FAO. FAOSTAT. Food and Agriculture Organization of the United Nations, Rome, Italy, 2015.
- CBN. Agricultural Development: Issues of Sustainability. In: O.J.; Nannna, S.O. Alade and E.O. Odoko (Eds.): Contemporary Economic Policy Issues in Nigeria, CBN Publications, 2003, pp. 185–222.
- Sharrock, S.; Frison, E. Musa production around the world: trends, varieties and regional importance. INIBAP Annual Report, 1998, pp. 42–47.
- FAO. Banana Market Review 2017. Food and Agriculture Organization of the United Nations, 2018, pp. 1–14.
- Nigel Amaya. The World's Leading Plantain Producers. https://www.worldatlas.com/articles/the-world-s- leading-plantain-producers/the-worlds-leading-plantain-producers, 2018, pp. 1-6.