Under healthy conditions, pancreatic β-cells produce and secrete the insulin hormone in response to blood glucose levels. Under diabetic conditions, however, β-cells are compelled to continuously secrete larger amounts of insulin to reduce blood glucose levels, and thereby, the β-cell function is debilitated in the long run.
- pancreatic β-cell dysfunction,incretin signaling
1. MafA and PDX-1 Play a Crucial Role in Pancreatic β-Cells
Under healthy conditions, pancreatic β-cells function to produce and secrete the insulin hormone in response to high glucose concentrations. Under diabetic conditions, however, β-cells are compelled to secrete larger amounts of insulin continuously in order to reduce blood glucose levels. Such succession is some grueling work for β-cells themselves, and thereby, the β-cell function is debilitated in the long run (so called “pancreatic β-cell glucose toxicity”) [1,2,3]. Chronic hyperglycemia reduces insulin biosynthesis and secretion together with reduced expression of insulin gene transcription factors such as MafA and PDX-1. In clinical practice, it is very important to alleviate such β-cell glucose toxicity so that the aggravation of diabetes mellitus is forestalled. In addition, insulin signaling in insulin target tissues (liver, skeletal muscle, and adipose tissue) is weakened by the burden of glucose toxicity, leading to the development of insulin resistance. Such debilitation of the β-cell function and development of insulin resistance lead to further aggravation of type 2 diabetes mellitus (Figure 1).
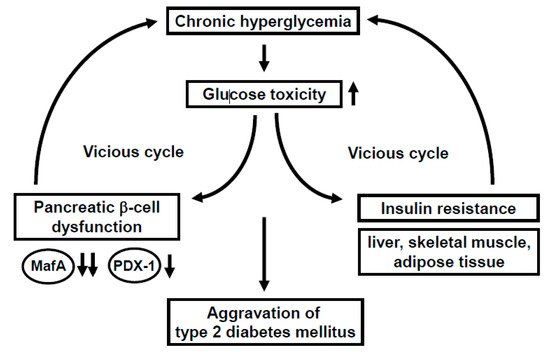
MafA is a strong transcription factor for the insulin gene [4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19]. In MafA knockout mice, insulin biosynthesis and secretion are reduced, leading to diabetes mellitus, indicating the importance of MafA in β-cells [8]. The MafA expression level is markedly reduced in a diabetic state [11], but it is preserved after alleviation of glucose toxicity with some anti-diabetic agent [16,17,18,19]. Furthermore, we reported that in β-cell-specific and conditional (tamoxifen-induced) MafA overexpressing transgenic db/db mice, serum insulin levels were higher and blood glucose levels were lower compared to their littermates. In addition, the β-cell mass was preserved in MafA overexpressing db/db mice mainly due to the reduction of apoptotic cell death. We think that these findings clearly underscore the importance of MafA in β-cells and become unequivocal evidence that downregulation of MafA expression is associated with β-cell glucose toxicity (Figure 1).
PDX-1, another important transcription factor for the insulin gene, plays a crucial role in pancreas development and β-cell differentiation and maintenance of mature β-cell function [20,21,22,23,24,25,26,27,28,29,30,31]. In PDX-1 knockout mice, pancreas formation is not observed at all [23]. In mature β-cells, PDX-1 transactivates several genes including insulin, glucokinase, and glut2. However, the expression level of PDX-1 is reduced in a diabetic state [26,29], which we think is associated with β-cell failure observed in diabetes. Indeed, we recently reported that β-cell-specific and conditional (tamoxifen-induced) PDX-1 overexpressing transgenic Akita mice showed lower blood glucose levels and lower HbA1C values compared to their littermates, accompanied by increased insulin secretion after glucose loading [31]. Expression levels of MafA and glucokinase were preserved in PDX-1 overexpressing Akita mice. We think that such new findings suggest that the downregulation of PDX-1 expression found in diabetes undermines insulin biosynthesis and secretion which explains, at least in part, the mechanism for β-cell glucose toxicity (Figure 1).
2. Incretin Signaling Plays an Important Role in Pancreatic β-Cells
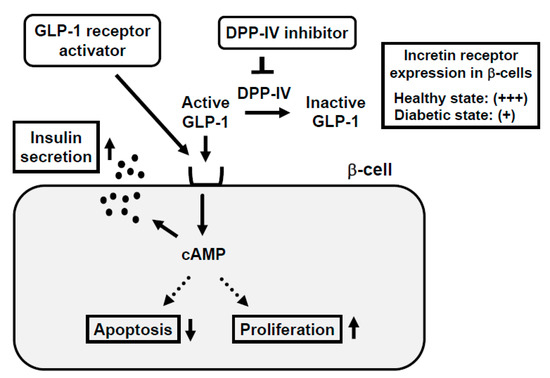
3. Incretin Receptor Expression in β-Cells Is Downregulated under Diabetic Conditions: Incretin-Based Agents Exert More Protective Effects on β-Cells at an Early Stage of Diabetes rather than an Advanced Stage
4. Impaired Insulin Signaling in Endothelial Cells Leads to Pancreatic β-Cell Dysfunction
In general, the main insulin target tissues are the liver, skeletal muscle, and adipose tissues, but there are many kinds of tissues and/or cells in which insulin signaling plays some important role. In endothelial cells, binding of insulin to insulin receptor on its cell surface activates insulin receptor substrate (IRS), phosphoinositide 3-kinase (PI3K), and 3-phosphoinositide-dependent protein kinase-1 (PDK1). Such activated insulin signaling leads to augment nitric oxide production in endothelial cells. Indeed, there have been several reports showing the importance of insulin signaling in endothelial cells [42,43,44,45,46]. Since it is known that the endothelial cell dysfunction is observed under diabetic conditions, it is possible that such endothelial dysfunction brings out hypoxia and ischemia in various tissues through insufficiency of nitric oxide production. In addition, it is known that pancreatic islets are particularly vulnerable to various stimuli including hypoxia and ischemia, and thereby it is also possible that endothelial dysfunction leads to exacerbation of pancreatic β-cell function.
Recently, we examined the possible role of PDK1, one of the important molecules in insulin signaling in vascular endothelial cells, in the maintenance of pancreatic β-cell mass and function. As a result, vascular endothelial-specific PDK1 knockout mice presented reduced β-cell mass and impaired β-cell function [47] (Figure 3). These mice also presented reduced blood flow of pancreas and/or islets and hypoxia of β-cells. In these KO mice, the β-cell mass was significantly reduced and the blood vessel region in islets was significantly decreased. In addition, in these KO mice, incretin secretion was augmented after the oral glucose tolerance test but insulin secretion was impaired, suggesting the impairment of incretin sensitivity in islets of KO mice. Insulin, MafA, PDX-1, GLP-1, and GIP receptor expression levels were all significantly decreased in islets of KO mice [47]. The microsphere experiment elucidated the remarkably reduced islet blood flow, and HIF1α and its downstream factor expression levels were significantly increased in islets of KO mice, indicating that islets of KO mice were in a more hypoxic state compared to the control mice. Consequently, ER stress-related gene expression levels were significantly elevated and inflammatory cytokine levels were increased in islets of KO mice [47].

Taken together, ablation of endothelial PDK1 reduces vascularity in islets, and both pancreatic and islet blood flow are decreased, which lead to hypoxia in islets and induction of ER stress and inflammation. Therefore, it is likely that vascular endothelial PDK1 plays an important role in the maintenance of pancreatic β-cell mass and function by maintaining the vascularity of the pancreas and islets and protecting them from hypoxia, hypoxia-related ER stress, and inflammation. These are novel concepts to explain the underlying molecular mechanism for pancreatic β-cell failure and we think that such findings would be useful when we think about future strategies for type 2 diabetes mellitus (Figure 3).
This entry is adapted from the peer-reviewed paper 10.3390/ijms21249444
