Aquaporins constitute a group of water channel proteins located in numerous cell types. These are pore-forming transmembrane proteins, which mediate the specific passage of water molecules through membranes. It is well-known that water homeostasis plays a crucial role in different reproductive processes, e.g., oocyte transport, hormonal secretion, completion of successful fertilization, blastocyst formation, pregnancy, and birth. Further, aquaporins are involved in the process of spermatogenesis, and they have been reported to be involved during the storage of spermatozoa. It is noteworthy that aquaporins are relevant for the physiological function of specific parts in the female reproductive system.
- Aquaporins
- Female Reproductive System
1. Introduction
An important milestone in the study of water fluxes through biological membranes was the discovery of an aqueous pore serving as a specific water channel (Figure 1), today, known as aquaporin (AQP). In 1992, Peter Agre et al. described this structure for the first time in erythrocytes, and it was termed Aquaporin-1 (AQP1) and proved to be a paradigm shift in the knowledge of molecular and trans-membrane water transport [1]. Since water is the main and essential component in a wide variety of cells, AQPs are very important since they are able to increase the water permeability of cell membranes [1][2]. Water movements through cell membranes are important features for the osmoregulation and water homeostasis of a cell [3][4]. It is well-known that biological membranes with their hydrophobic character of the lipid bilayer have an intrinsic permeability for water due to their lipid composition [5]. Since the first discovery of AQP1 until today, a total of thirteen AQP isoforms have been identified in humans (AQP0–12) and are all classified as membrane channels that contribute to the permeation of water through membranes, due to osmotic gradients [6][7]. To date, the mRNA or protein expression of the thirteen human aquaporin isoforms have been described in numerous organs and tissues. The classification of human AQPs into three groups is based on the primary structure and permeation abilities of AQPs [8][9][10]. In general, the architecture of AQPs in cell membranes can be described as homo tetrameric and each monomer constitutes a pore, which is functionally independent [11]. The discovery of AQPs started with ground-breaking experiments in 1992, where a glycosylated component of a 35–60 kD protein of human erythrocytes was described on the electrophoretogram. Only few years later, a new integral membrane protein of human erythrocytes was described, which was composed out of a non-glycosylated component (28 kD) and a glycosylated component (35–60 kD). This functional unit of a membrane water transporter was named “CHIP28” (channel-forming integral protein). However, in 1993, CHIP28 was renamed AQP1 by Agre et al., who were Nobel Prize laureates in chemistry for the discovery of water channels [12][13] in 2003. Since that time, over the last three decades, AQPs have been described as being present in several organ systems, and in this paper, their importance for the female reproductive system is elaborated.
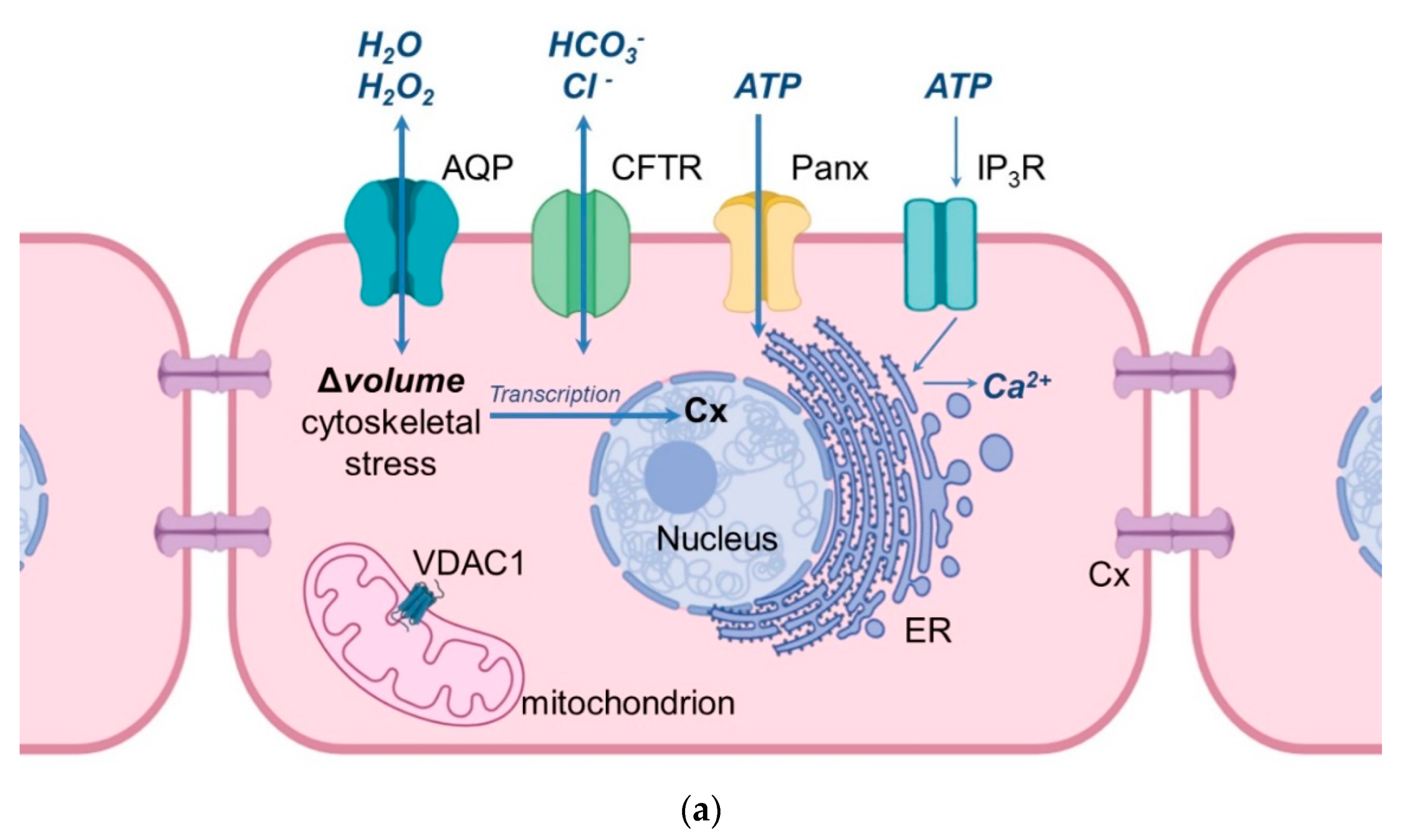
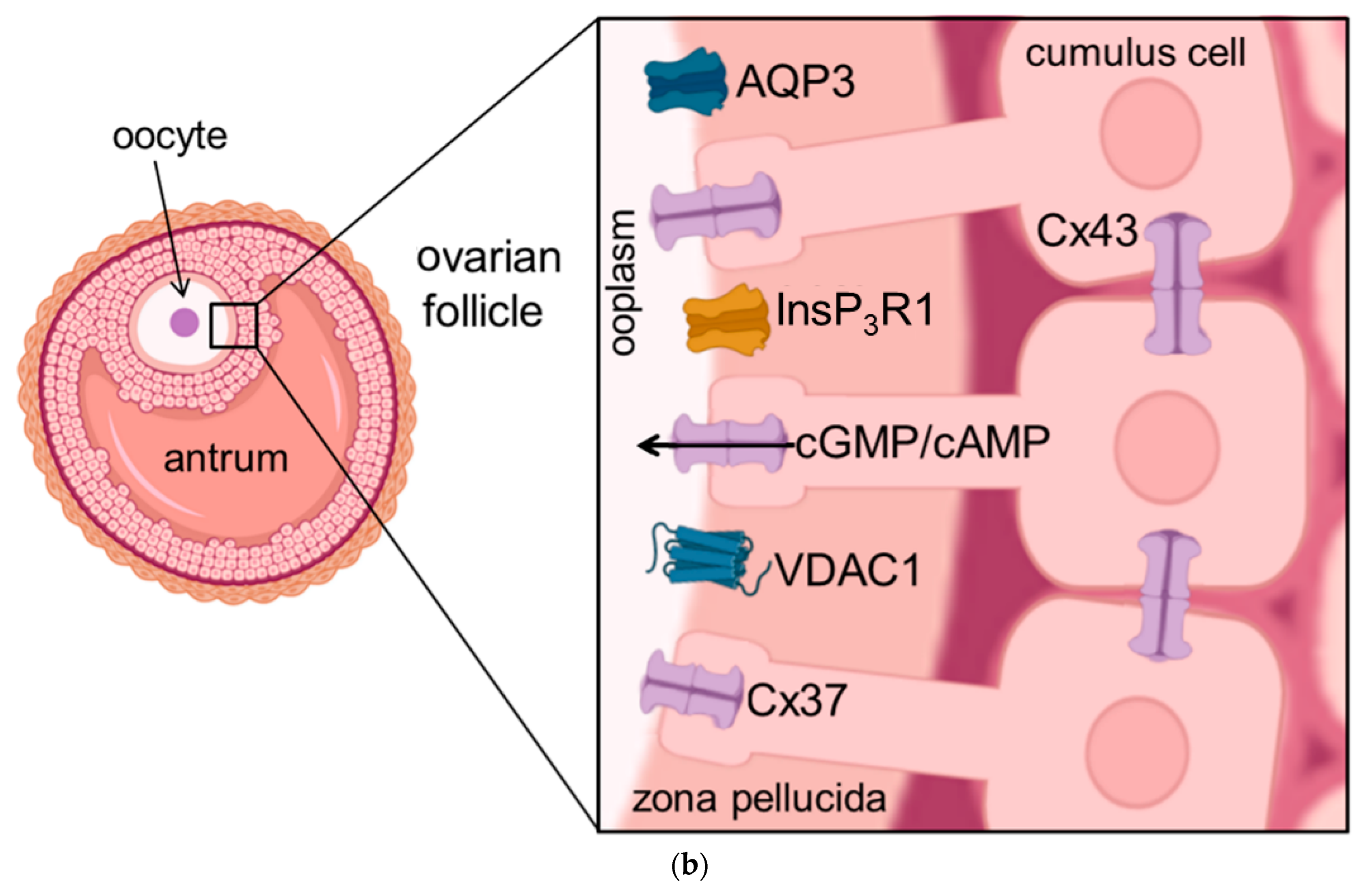
Figure 1. Schematic illustration of the cellular location of aquaporins and related proteins. (a) shows the localization and function of aquaporins and related proteins; (b) shows the localization of aquaporin and related proteins in the oocyte-cumulus-complex. Abbreviations: AQP—aquaporin; Cx—Connexin; CFTR—Cystic Fibrosis Transmembrane Conductance Regulator; Panx—Panexin; InsP3R1—Inositol trisphosphate receptor 1; VDAC1—Voltage dependent anion channel 1; ER—Endoplasmatic Reticulum; cGMP—cyclic Guanine Monophosphate; cAMP—cyclic Adenosine Monophosphate, ATP—Adenosine Triphosphate.
2. Aquaporins in the Female Mammalian Reproductive System
Previous studies have provided strong evidence, that at least eleven aquaporin isoforms, i.e., AQP 1, 2, 3, 4, 5, 6, 7, 8, 9, 11, and 12, have been identified in the female reproductive tract of different mammals, including the human, ovine, canine, and porcine species, and rodents. The first aquaporin in the female reproductive system was confirmed by isolating the complementary DNA encoding for a water channel generated from a human uterus. In this first report, the cloned cDNA appeared with a high (99.8%) homology to the 28 kDa human erythrocyte CHIP28 which was earlier mentioned [13]. Further, Li et al., who investigated the cDNAs of CHIP28 and uterus AQP, showed that the primary structures deduced from the cDNAs show 99% identity and the only difference is an alanine to valine substitution at position 45 of the human CHIP28 [14][15]. Some years later, the localization of AQP1 in rat uterine tissue was confirmed by mRNA expression [16]. In general, aquaporins in the female reproductive system appear to be involved in water movement at an intraluminal, interstitial, and capillary level, and their expression seems to be regulated by steroid sex hormones e.g., progesterone [17][18][19][20]. Due to these results provided by numerous research groups, aquaporins appear to be important for the female reproductive physiology, which will be discussed in the following section in a more detailed fashion.
2.1. The Expression of Aquaporins in the Vagina
As shown in Table 1, the abundance of AQP1–6 and AQP10–12 has been so far reported in the vagina, and the main role of AQPs in this part of the female reproductive tract is considered to be vaginal lubrication [21][22]. In pre-menopausal women, AQP1 appeared to be mainly localized (after immune-labelling) in the small blood vessels of the vaginal wall, i.e., in the capillaries and venules [21]. The proteins AQP2, 5, and 6 were immuno-localized in the cytoplasm of the vaginal epithelium, whereas the AQP3 protein was mainly detected in the plasma membrane of the vaginal epithelium [21]. Further, AQPs have also been detected in rat vagina [23][22]. When compared to the human species, rat AQPs show similar characteristics: similar protein localization, AQP1 in the rat vagina is localized in small blood vessels of the vaginal wall, AQP2 was detected in the cytoplasm of the vaginal epithelium, and AQP3 was also immune-localized in the plasma membrane of the vaginal epithelium [23][22]. Another study on intermediate layer cells of the murine vaginal epithelium provided strong evidence that AQP3 was detected in their plasma membrane [24]. Additionally, AQP4 was immune-localized in the basolateral membrane of superficial layer cells in the murine vaginal epithelium [24]. In summary, aquaporins appear to be mainly relevant for the moisture environment of the vaginal mucosa.
2.2. Aquaporins and the Functioning of the Ovary
Interestingly, AQP1 localization in the ovary is comparable to its localization in the vagina, i.e., in the microvascular and in the epithelial cells of small blood vessels, and its expression is rarely present in ovarian tumor cells [25][26]. The relative mRNA abundance for AQP1, 2, 3, and 4 was investigated in human ovarian follicles. More precisely, the expression of these four AQPs was present in theca and granulosa cells (GC) and their expression seemed to be dependent on the time to ovulation [27]. Therefore, it was assumed that the relative mRNA expression of AQP1–4 in the human ovary is controlled by ovarian hormones. Furthermore, a previous study provided evidence that AQP7–9 are also expressed in ovarian follicles of rats, where they most likely play a role during follicular development since AQPs seem to be responsible for the trans-cellular movement of H2O to form the antrum in antral follicles [28]. It was also shown that the mRNA expression of AQP5, 7, 8, 11, and 12 was detectable not only in neonatal murine ovaries, but also in murine GC of pups at the age of four weeks [29][30]. Further, the expression of mRNA and proteins has been reported for AQP5, 8, and 9, which appeared to be localized in the epithelium of rat oviducts, and, more specifically, the immune-localization for AQP5 and 8 was revealed in the cytoplasm, and AQP9 was localized in the plasma membrane [31]. AQP1, 5, and 9 have been demonstrated in the porcine female reproductive system, i.e., in the ovary, oviduct, and uterus [32][33][34][35]. Interestingly, AQP1 was detected in the endothelium of the ovarian capillaries, whereas AQP5 expression was analyzed in cells of primordial follicles, in GC of developing follicles, and epithelial cells of the oviduct [32]. In this part of the female reproductive tract aquaporins appear to be mainly involved in the supply of fluid, which is crucial for follicular development and growth according to the physiological function of the estrous cycle.
2.3. Aquaporins and the Functioning of the Uterus
The relevance of AQPs for the physiological function of the mammalian uterus as the crucial female organ of the reproductive tract is linked again to the vasculature, as already shown in other organs of this tract. The uterus is the major organ involved in feto-maternal communication, and fluid homeostasis during implantation, pregnancy, and early embryonic development [3]. AQP1 is highly expressed in the endothelium of uterine blood vessels [36]. Interestingly, AQP1 gene expression is much more abundant in capillaries and arteries compared to the same size veins of endometrial vasculature in women [37]. Contrary to the localization of AQP1, the expression of AQP2 was present in the glandular endometrial cells generated from women with physiological fertility [38]. Further, AQP3 was also reported to be expressed in the endometrium of women [39], and AQP3 was also highly abundant in human cervical cancer [40], but this will be presented in a more detailed fashion in the section related to female disorders. It has been reported that the AQP9 protein was localized in the cytoplasm of human oviductal epithelial cells [41]. Other studies have shown the expression of AQP1, 5, and 9 in the porcine uterus [33] and porcine oviduct [34] at different estrous cycle stages, namely at days 2–4, at days 10–12, and at days 14–16. Further, in the late stage of estrous cycle (days 18–20), there was also an expression detectable of AQP1, 5, and 9 in the porcine uteri [33] and oviducts [34]. It has also been assumed that the expression dynamics of AQP1, 5, and 9 in pigs appear to be influenced by the stages of the estrous cycle and early pregnancy due to hormonal composition [33][34][35]. Taking into account the uterine fluid homeostasis during the time of embryonic implantation, a fluid reduction has to take place during this crucial time to ensure the close contact of the early embryo to the superficial cells of the endometrium [42]. With regards to this, AQP 5 and 9 mediate the absorption of glandular fluid [3]. After implantation, placentation is also a crucial biological process, and the relevance of AQPs during this process will be reviewed in the following section. To sum up, aquaporins appear to be mainly responsible for creating the proper fluid micro-environment in the uterus and they contribute to the lubrication of the endometrium, which is crucial for sperm movement and implantation.
2.4. Aquaporins and the Functioning of the Placenta
Numerous AQPs have been reported to be present in fetal membranes and are crucial during placentation and early stages of pregnancy. The haemochorialis placenta of the human species has shown a high relative mRNA abundance of AQP1, 3, 9, and 11 in the chorionic villi, whereas the mRNA abundance for AQP4, 5, and 8 was lower in the earlier mentioned part of the placenta [43]. Both gene and protein expression for AQP1, 3, 8, 9, and 11 have revealed the presence of these aquaporins in the human amnion and chorion during the entire length of pregnancy [44][45]. The relative mRNA abundance of AQP1 was reported to be in the placental vasculature [46] and AQP3 gene-expression was detected in the trophectoderm [45]. Further, the localization of the AQP3 protein in the human placenta has been reported in epithelial cells of the chorion and amnion [47]. Evidence has been provided that the expression of AQP4 was decreased in cells of the syncytiotrophoblast, but endothelial and stromal cells of placental villi collected in the first and third trimester of pregnancy showed an increase in AQP4 expression [48], which suggests that the expression of AQP4 appears to be pregnancy stage-dependent. AQP8 and AQP9 have been localized to the epithelium of the human amnion [49] and AQP9 was further shown to be present in trophoblast cells, in cytotrophoblast cells, and syncytiotrophoblast cells of the chorion [50][51]. During the phase of implantation and early placentation, the expression of AQP1, 5, and 9 have also been detected in the porcine species [33][34][35].
This entry is adapted from the peer-reviewed paper 10.3390/cells9122570
References
- Preston, G.M.; Carroll, T.P.; Guggino, W.B.; Agre, P. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science 1992, 256, 385–387.
- Mathai, J.C.; Agre, P. Hourglass pore-forming domains restrict aquaporin-1 tetramer assembly. Biochemistry 1999, 38, 923–928.
- Sha, X.Y.; Xiong, Z.F.; Liu, H.S.; Di, X.D.; Ma, T.H. Maternal-fetal fluid balance and aquaporins: From molecule to physiology. Acta Pharmacol. Sin. 2011, 32, 716–720.
- Ducza, E.; Csányi, A.; Gáspár, R. Aquaporins during pregnancy: Their function and significance. Int. J. Mol. Sci. 2017, 18, 2593.
- Törnroth-Horsefield, S.; Hedfalk, K.; Fischer, G.; Lindkvist-Petersson, K.; Neutze, R. Structural insights into eukaryotic aquaporin regulation. FEBS Lett. 2010, 584, 2580–2588.
- Agre, P.; King, L.S.; Yasui, M.; Guggino, W.B.; Ottersen, O.P.; Fujiyoshi, Y.; Engel, A.; Nielsen, S. Aquaporin water channels—From atomic structure to clinical medicine. J. Physiol. 2002, 542 Pt 1, 3–16.
- Huang, H.F.; He, R.H.; Sun, C.C.; Zhang, Y.; Meng, Q.X.; Ma, Y.Y. Function of aquaporins in female and male reproductive systems. Hum. Reprod. Update 2006, 12, 785–795.
- Yasui, M.; Kwon, T.H.; Knepper, M.A.; Nielsen, S.; Agre, P. Aquaporin-6: An intracellular vesicle water channel protein in renal epithelia. Proc. Natl. Acad. Sci. USA 1999, 10, 5808–5813.
- Saparov, S.M.; Liu, K.; Agre, P.; Pohl, P. Fast and selective ammonia transport by aquaporin-8. J. Biol. Chem. 2007, 282, 5296–5301.
- Tsukaguchi, H.; Shayakul, C.; Berger, U.V.; Mackenzie, B.; Devidas, S.; Guggino, W.B.; Van Hoek, A.N.; Hediger, M.A. Molecular characterization of a broad selectivity neutral solute channel. J. Biol. Chem. 1998, 273, 24737–24743.
- Ozu, M.; Galizia, L.; Acuña, C.; Amodeo, G. Aquaporins: More Than Functional Monomers in a Tetrameric Arrangement. Cells 2018, 7, 209.
- Benga, G.; Popescu, O.; Borza, V.; Pop, V.I.; Muresan, A.; Mocsy, I.; Brain, A.; Wrigglesworth, J.M. Water permeability in human erythrocytes: Identification of membrane proteins involved in water transport. Eur. J. Cell Biol. 1986, 41, 252–262.
- Agre, P.; Preston, G.M.; Smith, B.L.; Jung, J.S.; Raina, S.; Moon, C.; Guggino, W.B.; Nielsen, S. Aquaporin CHIP: The archetypal molecular water channel. Am. J. Physiol. Ren. Fluid Electrolyte Physiol. 1993, 265 Pt 2, F463–F476.
- Day, R.E.; Kitchen, P.; Owen, D.S.; Bland, C.; Marshall, L.; Conner, A.C.; Bill, R.M.; Conner, M.T. Human aquaporins: Regulators of transcellular water flow. Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 1492–1506.
- Li, X.; Yu, H.; Koide, S.S. The water channel gene in human uterus. Biochem. Mol. Biol. Int. 1994, 32, 371–377.
- Li, X.J.; Yu, H.M.; Koide, S.S. Regulation of water channel gene (AQP-CHIP) expression by estradiol and anordiol in rat uterus. Yaoxue Xuebao 1997, 32, 586–592.
- Ferré-Dolcet, L.; Yeste, M.; Vendrell, M.; Rigau, T.; Rodríguez-Gil, J.E.; del Alamo, M.M.R. Uterine and placental specific localization of AQP2 and AQP8 is related with changes of serum progesterone levels in pregnant queens. Theriogenology 2020, 142, 149–157.
- Jablonski, E.M.; McConnell, N.A.; Hughes, F.M.; Huet-Hudson, Y.M. Estrogen Regulation of Aquaporins in the Mouse Uterus: Potential Roles in Uterine Water Movement. Biol. Reprod. 2003, 69, 1481–1487.
- Richard, C.; Gao, J.; Brown, N.; Reese, J. Aquaporin water channel genes are differentially expressed and regulated by ovarian steroids during the periimplantation period in the mouse. Endocrinology 2003, 144, 1533–1541.
- Lindsay, L.A.; Murphy, C.R. Redistribution of aquaporins 1 and 5 in the rat uterus is dependent on progesterone: A study with light and electron microscopy. Reproduction 2006, 131, 369–378.
- Kim, S.O.; Oh, K.J.; Lee, H.S.; Ahn, K.; Kim, S.W.; Park, K. Expression of aquaporin water channels in the vagina in premenopausal women. J. Sex. Med. 2011, 8, 1925–1930.
- Zhu, J.; Xia, J.; Jiang, J.; Jiang, R.; He, Y.; Lin, H. Effects of estrogen deprivation on expression of aquaporins in rat vagina. Menopause 2015, 22, 893–898.
- Park, K.; Han, H.J.; Kim, S.W.; Jung, S.I.; Kim, S.O.; Lee, H.S.; Lee, M.N.; Ahn, K. Expression of aquaporin water channels in rat vagina: Potential role in vaginal lubrication. J. Sex. Med. 2008, 5, 77–82.
- Yin, Y.; Lin, C.; Ma, L. Msx2 promotes vaginal epithelial differentiation and Wolffian duct regression and dampens the vaginal response to diethylstilbestrol. Mol. Endocrinol. 2006, 20, 1535–1546.
- Yang, J.H.; Shi, Y.F.; Cheng, Q.; Qian, Y.L. Protein and mRNA expression of aquaporin-1 in epithelial ovarian tumors and its clinic significance. Zhonghua Fu Chan Ke Za Zhi 2005, 40, 623–626.
- Yang, J.H.; Shi, Y.F.; Chen, X.D.; Qi, W.J. The influence of aquaporin-1 and microvessel density on ovarian carcinogenesis and ascites formation. Int. J. Gynecol. Cancer 2006, 16 (Suppl. S1), 400–405.
- Thoroddsen, A.; Dahm-Kähler, P.; Lind, A.K.; Weijdegard, B.; Lindenthal, B.; Müller, J.; Brännström, M. The water permeability channels aquaporins 1-4 are differentially expressed in granulosa and theca cells of the preovulatory follicle during precise stages of human ovulation. J. Clin. Endocrinol. Metab. 2011, 96, 1021–1028.
- McConnell, N.A.; Yunus, R.S.; Gross, S.A.; Bost, K.L.; Clemens, M.G.; Hughes, F.M. Water permeability of an ovarian antral follicle is predominantly transcellular and mediated by aquaporins. Endocrinology 2002, 143, 2905–2912.
- Su, W.; Guan, X.; Zhang, D.; Sun, M.; Yang, L.; Yi, F.; Hao, F.; Feng, X.; Ma, T. Occurrence of multi-oocyte follicles in aquaporin 8-deficient mice. Reprod. Biol. Endocrinol. 2013, 11, 88.
- West-Farrell, E.R.; Xu, M.; Gomberg, M.A.; Chow, Y.H.; Woodruff, T.K.; Shea, L.D. The mouse follicle microenvironment regulates antrum formation and steroid production: Alterations in gene expression profiles. Biol. Reprod. 2009, 80, 432–439.
- Brañes, M.C.; Morales, B.; Ríos, M.; Villalón, M.J. Regulation of the immunoexpression of aquaporin 9 by ovarian hormones in the rat oviductal epithelium. Am. J. Physiol. Cell Physiol. 2005, 288, C1048–C1057.
- Skowronski, M.T.; Kwon, T.H.; Nielsen, S. Immunolocalization of aquaporin 1, 5, and 9 in the female pig reproductive system. J. Histochem. Cytochem. 2009, 57, 61–67.
- Skowronski, M.T. Distribution and quantitative changes in amounts of aquaporin 1, 5 and 9 in the pig uterus during the estrous cycle and early pregnancy. Reprod. Biol. Endocrinol. 2010, 8, 109.
- Skowronski, M.T.; Skowronska, A.; Nielsen, S. Fluctuation of aquaporin 1, 5, and 9 expression in the pig oviduct during the estrous cycle and early pregnancy. J. Histochem. Cytochem. 2011, 59, 419–427.
- Skowronski, M.T.; Frackowiak, L.; Skowronska, A. Expression of aquaporin 1 in the pig peri-ovarian vascular complex during the estrous cycle and early pregnancy. Reprod. Biol. 2011, 11, 210–223.
- Feng, C.; Sun, C.C.; Wang, T.T.; He, R.H.; Sheng, J.Z.; Huang, H.F. Decreased expression of endometrial vessel AQP1 and endometrial epithelium AQP2 related to anovulatory uterine bleeding in premenopausal women. Menopause 2008, 15, 648–654.
- Hildenbrand, A.; Stavreus-Evers, A.; Lalitkumar, P.G.L.; Nielsen, S.; Mints, M.; Gemzell-Danielsson, K. Aquaporin 1 is expressed in the human endometrium during normal cycle and increases after mifepristone treatment. Int. J. Mol. Med. 2008, 22, 49–53.
- Hildenbrand, A.; Lalitkumar, L.; Nielsen, S.; Gemzell-Danielsson, K.; Stavreus-Evers, A. Expression of aquaporin 2 in human endometrium. Fertil. Steril. 2006, 86, 1452–1458.
- Mobasheri, A.; Wray, S.; Marples, D. Distribution of AQP2 and AQP3 water channels in human tissue microarrays. J. Mol. Histol. 2005, 36, 1–14.
- De Wilde, J.; Wilting, S.M.; Meijer, C.J.L.M.; Van De Wiel, M.A.; Ylstra, B.; Snijders, P.J.F.; Steenbergen, R.D.M. Gene expression profiling to identify markers associated with deregulated hTERT in HPV-transformed keratinocytes and cervical cancer. Int. J. Cancer 2008, 122, 877–888.
- Ji, Y.F.; Chen, L.Y.; Xu, K.H.; Yao, J.F.; Shi, Y.F.; Shanguan, X.J. Reduced expression of aquaporin 9 in tubal ectopic pregnancy. J. Mol. Histol. 2013, 44, 167–173.
- Enders, A.C.; Schlafke, S. A morphological analysis of the early implantation stages in the rat. Am. J. Anat. 1967, 120, 185–225.
- Escobar, J.; Gormaz, M.; Arduini, A.; Gosens, K.; Martinez, A.; Perales, A.; Escrig, R.; Tormos, E.; Roselló, M.; Orellana, C.; et al. Expression of aquaporins early in human pregnancy. Early Hum. Dev. 2012, 88, 589–594.
- Prat, C.; Blanchon, L.; Borel, V.; Gallot, D.; Herbet, A.; Bouvier, D.; Marceau, G.; Sapin, V. Ontogeny of aquaporins in human fetal membranes. Biol. Reprod. 2012, 86, 48.
- Zhu, X.Q.; Jiang, S.S.; Zhu, X.J.; Zou, S.W.; Wang, Y.H.; Hu, Y.C. Expression of Aquaporin 1 and Aquaporin 3 in Fetal Membranes and Placenta in Human Term Pregnancies with Oligohydramnios. Placenta 2009, 30, 670–676.
- Štulc, J. Placental transfer of inorganic ions and water. Physiol. Rev. 1997, 77, 805–836.
- Wang, S.; Amidi, F.; Beall, M.; Gui, L.; Ross, M.G. Aquaporin 3 Expression in Human Fetal Membranes and its Up-regulation by Cyclic Adenosine Monophosphate in Amnion Epithelial Cell Culture. J. Soc. Gynecol. Investig. 2006, 13, 181–185.
- De Falco, M.; Cobellis, L.; Torella, M.; Acone, G.; Varano, L.; Sellitti, A.; Ragucci, A.; Coppola, G.; Cassandro, R.; Laforgia, V.; et al. Down-regulation of aquaporin 4 in human placenta throughout pregnancy. In Vivo 2007, 21, 813–817.
- Liu, H.S.; Hao, R.Z.; Song, X.F.; Xiong, Z.F. Aquaporin 8 expression in human placenta and fetal membrane. J. Clin. Rehabil. Tissue Eng. Res. 2009, 28, 333–336.
- Wang, S.; Chen, J.; Beall, M.; Zhou, W.; Ross, M.G. Expression of aquaporin 9 in human chorioamniotic membranes and placenta. Am. J. Obstet. Gynecol. 2004, 191, 2160–2167.
- Zhu, X.; Jiang, S.; Hu, Y.; Zheng, X.; Zou, S.; Wang, Y.; Zhu, X. The expression of aquaporin 8 and aquaporin 9 in fetal membranes and placenta in term pregnancies complicated by idiopathic polyhydramnios. Early Hum. Dev. 2010, 86, 657–663.