Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Clinical Neurology
Sporadic inclusion body myositis (sIBM) is the most common muscle disease of older people and is clinically characterized by slowly progressive asymmetrical muscle weakness, predominantly affecting the quadriceps, deep finger flexors, and foot extensors.
- sporadic inclusion body myositis (sIBM)
- inflammation
- aging
- inflammaging
1. Introduction
Sporadic inclusion body myositis (sIBM) is a chronic progressive muscle disease that primarily affects people 50 years and older [1,2]. It is the most common acquired myopathy in the elderly, with a prevalence ranging between 5 and 180 per million, depending on the geographical area [3,4,5,6]. sIBM is more common in males than females (2:1) and is associated with higher morbidity and mortality [5,6]. The disease presents with muscle weakness mainly affecting the quadriceps and finger flexors [6]. At present, there are no disease-modifying therapies for this progressive disease that eventually leads to severe disability [7]. Despite common clinical characteristics, the phenotype can be variable, and the diagnosis relies on the combination of clinical evaluation, laboratory tests, and pathologic changes in muscle biopsy [6]. Histological hallmarks include both degenerative features such as protein aggregation in myofibers and autoimmune aspects such as endomysial infiltration by T cells [8,9].
sIBM is classified among the idiopathic inflammatory myopathies (IIM) along with dermatomyositis (DM), polymyositis (PM), and immune-mediated necrotizing myopathy (IMNM), but the lack of response to immunosuppressive treatment by sIBM patients have raised questions about the relevance of immune processes in disease pathogenesis [8,10,11].
2. Diagnosis of sIBM
2.1. Clinical Aspects
sIBM is clinically characterized by slowly progressive asymmetrical muscle weakness, predominantly affecting the quadriceps, deep finger flexors, and foot extensors [1,8]. Pharyngeal muscles often become impaired, resulting in dysphagia [12]. Head drop and camptocormia may occur, and facial muscles are occasionally affected [13,14]. Late stages of the disease are characterized by weakness and atrophy of distal and proximal muscles and possible impairment of neck flexors and extensors [15]. Sensory function is normal, and deep tendon reflexes are preserved, but when atrophy of major muscles occurs, the latter can be abolished [16]. Disease progression is usually slow. Variability in the clinical presentation and a lack of specific features/muscle involvement in some patients represent challenges for a prompt and correct diagnosis, making laboratory tests and histological analysis of muscle biopsy fundamental tools in the diagnostic workup [7].
2.2. Laboratory Studies
CK levels are frequently normal or only mildly elevated, usually less than 10 times the upper normal values [9,17].
Autoantibodies are considered a useful tool in the diagnosis of IIM. Based on their specificity, autoantibodies in IIM are traditionally classified as myositis-specific (MSA) or myositis-associated antibodies (MAA), with the latter also occurring in other systemic autoimmune rheumatic diseases [18]. In 2011, Salajegheh et al. showed that autoantibodies targeting a ~44 kDa human muscle protein (referred to as Mup44) occur in the serum of 52% of patients with sIBM (n = 25) with 100% specificity for the disease [19]. In 2013, two independent studies identified Mup44 as cytosolic 5′-nucleotidase 1A (cN1A), which is an enzyme highly expressed in skeletal muscle that catalyzes the dephosphorylation of adenosine monophosphate into adenosine and phosphate [20,21]. Even if absent or extremely rare in healthy controls, antibodies against cN1A have also been detected in primary Sjögren’s syndrome (pSS) and systemic lupus erythematosus (SLE), making them not specific for sIBM [22,23]. Nevertheless, anti-cN1A antibodies are considered a valuable diagnostic biomarker for sIBM because they occur in 33 to 76% of patients with sIBM and in less than 5% of patients with PM, DM, or non-autoimmune neuromuscular diseases [23]. It has been reported that the specificity of anti-cN1A to distinguish sIBM from other IIM, neuromuscular diseases, and autoimmune disorders ranges between 87 and 100% and between 74.6 and 92% to discriminate sIBM from other types of IIM [24]. Contradictory observations have been made regarding the association of anti-cN1A antibodies with a more severe phenotype and increased mortality risk independent of age, gender, comorbidities, and the presence of dysphagia [20,23,24,25,26,27,28,29,30,31]. Even though there is no cure for sIBM, antibodies against cN1A are helpful in the diagnostic workup to reduce misdiagnosis with PM and consequent administration of steroids that worsen sIBM progression after discontinuation [10,15].
2.3. Magnetic Resonance Imaging (MRI)
In recent years, muscle MRI has emerged as an important tool in the diagnosis of muscle disorders due to its ability to highlight affected muscle by revealing atrophy, fat infiltration and signs of inflammation [32]. In sIBM, MRI changes have been reported in both upper and lower limbs, are more severe in distal segments, and usually have an asymmetric pattern [32,33,34,35]. Fat infiltration is commonly seen in MRI scans [33,35,36,37]. It has been observed a distinctive MRI pattern that can discriminate between sIBM and other myopathies, namely the relative sparing of the rectus femoris, hamstrings, and adductor muscles and the extent and asymmetry of fatty infiltration, mostly seen in the adductor magnus [38]. Furthermore, flexor digitorum profundus seems to be involved in sIBM patients on imaging even before its weakness becomes clinically detectable [38].
2.4. Pathological Characteristics
sIBM muscle biopsy shows both degenerative and inflammatory features (Figure 1). Histopathological changes detectable by light microscopy include the following.
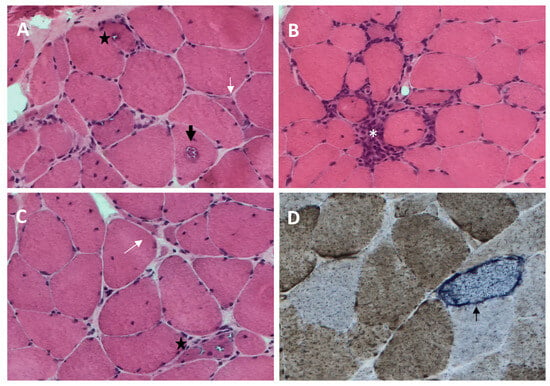
Figure 1. Histopathological features of sporadic inclusion body myositis (sIBM) muscle biopsy ((A–C), hematoxylin and eosin; (D), cytochrome c oxidase (COX)-succinate dehydrogenase): endomysial inflammatory infiltrate that invades nonnecrotic muscle fibers (white asterisk, (B)), rimmed vacuoles within muscle fibers (black star, (A,C)), eosinophilic cytoplasmic inclusion (black arrow, (A)), ragged red fiber and COX-negative fibers (thin black arrow, (D)), and angulated muscle fibers (white arrow, (A,C)).
- (1)
-
Rimmed vacuoles, namely irregular vacuoles of variable size and shape, bordered by basophilic granular deposits that occur in nonnecrotic muscle fibers. Rimmed vacuoles can be a rather rare finding and are visible in 0.4–6.4% of the fibers [9];
- (2)
-
Eosinophilic cytoplasmic inclusions visible at hematoxylin and eosin (H&E) and modified Gomori trichrome staining [17];
- (3)
-
Cytoplasmic accumulation of aggregated/misfolded proteins, referred to as amyloid deposits or protein inclusions, which occur in 60 to 80% of the sIBM vacuolated muscle fibers, usually in non-vacuolated areas of the fiber [1,17]. These β-pleated-sheet amyloid inclusions are detectable by fluorescence-enhanced Congo red staining. Several proteins have been found within these aggregates, including amyloid-β precursor protein (AβPP), amyloid-β (Aβ), phosphorylated-tau (p-tau), and ubiquitin, to name a few [8]. Notably, TDP-43 immunopositivity has been reported in over 60% of sIBM patients and is considered a hallmark of sIBM pathology [39,40];
- (4)
-
Endomysial lymphocytic infiltrates consisting predominantly of macrophages and CD8+T cells that invade nonnecrotic muscle fibers expressing MHC class I on the sarcolemma [17];
- (5)
-
Mitochondrial abnormalities consisting of the abnormal proliferation of mitochondrial leading to ragged red fibers (RRFs, muscle fibers containing excessive mitochondrial proliferation) and the impairment of mitochondrial function, as shown by increased cytochrome c oxidase (COX)-negative fibers [41,42];
- (6)
-
Angulated muscle fibers of small caliber suggesting a neurogenic process [43].
Ultrastructural analysis reveals characteristic degenerative changes such as the following:
- (1)
- (2)
-
Cytoplasmic 6–10 nm amyloid-like filaments containing Aβ, deposits of flocculomembranous and amorphous material [1].
2.5. Correlation of Pathological Features with Clinical Progression and Laboratory Findings
Overall, only a few studies have attempted to correlate specific clinical and/or laboratory findings with muscle histopathological changes. A recent retrospective study on 50 sIBM patients documented a strong and statistically significant positive correlation of endomysial inflammation with the severity of dysphagia and, conversely, a negative correlation with the modified Rankin scale (mRS) whereas the degenerative features such as rimmed vacuoles and congophilic inclusions showed no correlation with any of the clinical measures including muscle strength sum score, quadriceps strength, mRS and severity of dysphagia [44]. Varying and conflicting observations have been made regarding the association of anti-cN1A antibodies with pathological features, thus anti-cN1A antibodies correlated with fewer rimmed vacuoles [45], more cytochrome oxidase negative myofibers [25,46], a higher number of regenerating fibers [44], or auto-aggressive inflammation [47]. CK levels correlated with a broad spectrum of histological findings, including muscle fiber necrosis, regeneration, endomysial and perimysial inflammation, and increased endomysial connective tissue [44].
3. Inflammation in sIBM Pathogenesis
The involvement of the immune system in sIBM is supported by the presence of endomysial immune cell infiltration and circulating autoantibodies. Although the role of inflammation in the pathogenesis of sIBM has been under discussion for a long time, many studies have provided data to support the involvement of both innate and adaptive immunity in the disease.
3.1. T Cells
Infiltrating immune cells are mostly represented by cytotoxic CD8+T cells that surround non-necrotic muscle fibers and are often localized in proximity to MHCI-expressing myofibers [17]. It has been estimated that CD8+ T cells are about fivefold more abundant than CD4+ T cells in sIBM muscle [152]. Several studies have shown that CD8+ T cells have restricted expression of T cell receptor (TCR) Vα/Vβ genes in the blood and muscle of sIBM patients and are clonally expanded, suggesting that muscle-specific antigen drives the inflammatory response in the disease [153,154,155,156,157,158,159,160,161]. Interestingly, the expression of Vβ genes was found to be different in muscle-infiltrating lymphocytes and peripheral blood, indicating that T cells expand in situ in response to antigens presented by MHCI-expressing muscle cells, or they are selectively recruited to the muscle [156,157]. Identical T cell clones have been found in different muscles and persist for years, indicating that they continuously react to muscle antigens, perpetuating the inflammatory response over time [155,162].
T cell activation requires antigen presentation via MHC and the interaction between CD28 on T cells with costimulatory molecules on antigen-presenting cells (APCs). Expression of the costimulatory molecules BB-1, ICOS, and B7-H3 by muscle fibers has been demonstrated in sIBM and suggests that myofibers may present the antigen and activate T cells expressing the appropriate TCR [163,164,165,166].
Regarding phenotypic and functional features, infiltrating CD8+ T cells express markers of highly differentiated effector cells, have proinflammatory and cytotoxic features, and have minimal or no proliferative ability [158,167,168,169,170]. Specifically, expression of the cytolytic molecules perforin, granulysin, and granzyme B, and natural killer (NK) markers CD16 or CD56, have been detected in CD8+T cells invading skeletal muscle, indicating they share functional and phenotypic features of NK cells [165,171,172,173]. The occurrence of Th1 immune response has been supported by increased expression levels of the cytokines and chemokines CXCL-9, CXCL-10, IL-12, CCL-2, and IL-1RA and by a higher number of IFN-γ expressing CD8+CD28− T cells in the blood of patients with sIBM [169]. These findings indicate that cytotoxic T cells respond to yet unknown muscle antigen/s and contribute to the myofiber damage that occurs in sIBM [9].
On the other hand, several observations show that, in sIBM, T cells are highly differentiated and have reduced effector functions. In this regard, it has been found that peripheral blood and muscle infiltrating T cells are effector memory T (TEM) cells and terminally differentiated effector memory T (TEMRA), as indicated by the lack of expression of CD28 and by the acquisition of CD224 and CD57 expression [9,169,174]. Loss of CD28 and gain of CD57 in T cells are known to be associated with decreased proliferative capacity, terminal differentiation, senescence, and clonal exhaustion, a state occurring with chronic antigen stimulation [175,176]. Exhausted T-cells have decreased ability to respond to cytokines, have lost their effector functions, and have upregulated inhibitory receptors (IRs) such as programmed cell death protein1 (PD-1), lymphocyte activation gene-3 (LAG-3), T-cell immunoglobulin domain and mucin domain-3 (TIM-3), and T-cell immune receptor with Ig and ITIM domains (TIGIT) [174,177,178]. The main function of IRs is to negatively regulate the activation and the effector functions of T cells and are responsible for fine-tuning T cell activity [178]. IRs have a key role in controlling immune responses and establishing T cell tolerance [178]. The expression of PD-1 has been reported on T cells infiltrating sIBM muscle [179]. PD1 ligands PD-L1 and PD-L2 have been detected on infiltrating macrophages and on myofibers, respectively, and possibly form immunologic synapses with PD1 expressed by lymphocytes [179]. In support of an exhausted phenotype of T cells in sIBM, the mRNA levels of the exhaustion markers PD1, EOMES, TBX21, LAG3, CD244, TIM3, and KLRG1 were found to be increased in sIBM muscle [179]. Taken together, these data indicate that, in sIBM, persistent exposure to antigens drives muscle infiltrating T cells toward a state of exhaustion characterized by reduced or absent effector functions and proliferation ability. These observations suggest that the T cell-mediated cytotoxic damage of muscle fibers likely plays a role in the early stages of the disease before T cell exhaustion occurs.
Regulatory T cells (Tregs) represent a subpopulation of CD4+T cells which have the main function to suppress immune responses [180]. Their immune suppressive activity is crucial for keeping immune reactions against invading pathogens under control and maintaining immune tolerance toward self-antigens [180]. The decreased number and function of Tregs have been associated with loss of tolerance and autoimmune diseases [181]. Studies have demonstrated that Tregs are able to control local immune responses during regeneration after skeletal muscle injury [182,183]. Although Tregs suppression activity in sIBM was found to be normal, a reduction in their number was observed in the blood and skeletal muscle of sIBM patients and proposed as a factor contributing to the autoimmune response to skeletal muscle in this disease [169].
3.2. Plasma Cells and Antibody-Mediated Immune Response
Several studies have provided evidence for a muscle antigen-driven B- and plasma cell-mediated immune response and humoral immunity in sIBM. Differentiated CD138+ plasma cells, but not CD19+ or CD20+ B cells, and increased expression of immunoglobulin gene transcripts were detected in the skeletal muscle of patients with sIBM [184]. Analysis of the variable region of Ig H chain gene transcript obtained from sIBM muscles demonstrated that B cells and their descendent plasma cells undergo oligoclonal expansion, isotype switching, and accumulate somatic mutations [185]. These processes usually occur in secondary lymphoid organs where B cells migrate after exposure to antigens to maturate and differentiate into antibody-producing plasma cells [185]. Interestingly, it has been observed that in sIBM muscles, inflammatory infiltrates of T cells, B cells, plasma cells, and myeloid dendritic cells assemble in lymphoid structures that could support the in situ maturation of B cells into antibody-secreting plasma cells [186]. Furthermore, increased serum levels of B-cell activating factor (BAFF), a cytokine involved in the survival, maturation, and differentiation of B cells, were detected in the serum of patients with sIBM [187]. These findings indicate that local maturation of B cells to antibody-producing plasma cells occurs in the muscle of sIBM patients in response to muscle antigen/s [185]. In agreement with these observations, a recent study revealed the sIBM muscle B cell receptor (BCR) repertoire and showed that it has distinct features from those of other IIMs, indicating that it may originate from exposure to disease-specific antigens [188].
The presence of a humoral immune response in sIBM was further supported by the detection of antibodies against cN1A, as described previously in this manuscript. The pathogenic role of the anti-cN1A was supported by the finding that mice immunized with anti-cN1A IgG isolated from patients show increased sarcoplasmic aggregation of p62 and LC3, degenerative features of sIBM muscle [30].
Overall, these findings support that in sIBM, B cells become activated and mature into plasma cells that produce antibodies directed against a muscle antigen.
3.3. Macrophages and Dendritic Cells
Macrophages and myeloid dendritic cells (DCs) have also been detected in sIBM muscle [152,189]. Proteomic studies have shown that CD74, CD163, and STAT1 are increased in sIBM muscle, and immunohistochemical analysis revealed these molecules are enriched in macrophages [190]. Specifically, CD74 was found to be expressed in CD68+ macrophages and the sarcolemma. CD163 was detected in endomysial macrophages, and STAT1 was found in macrophages that displayed phagocytic activity [190]. The interferon-induced protein ISG15 and IRF8 were found to be strongly expressed on MHCII+ macrophages, indicating that these cells are in an activated state [190].
DCs are also infiltrating the sIBM muscle. A study of the two populations of DCs, namely myeloid DC (mDCs) and plasmacytoid DC (pDCs), revealed that mDCs surround and invade healthy myofibers [191,192]. The close colocalization of mDCs with T cells has been interpreted as a possible role of mDCs in antigen presentation to T cells [191,192].
These studies show that, despite the expression of MHC on muscle fibers of sIBM patients and their possible contribution to antigen presentation, professional antigen-presenting cells (APCs) also play a role in the pathogenesis of sIBM [193,194,195]. However, further efforts are needed to better characterize the phenotype and function of macrophages and dendritic cells in sIBM.
This entry is adapted from the peer-reviewed paper 10.3390/ijms25052742
This entry is offline, you can click here to edit this entry!
