The peripheral nervous system (PNS) has a considerable ability to regenerate following injury, and Schwann cells are essential players in the regulation of the nerve repair process. Schwann cells, the PNS myelinating glia, contribute to realizing the necessary extracellular microenvironment for axonal regrowth and restoration of myelin sheaths on regenerating axons [
1]. Following nerve injury, Schwann cells acquire a new phenotype and shut down the myelination process. To achieve nerve regeneration, these cells execute a complex repair program including formation of Bungner band, a regeneration track in the distal site of the injured nerve; production of cytokines that recruit macrophages for axonal and myelin debris clearance; synthesis and secretion of various neurotrophic factors [
2].
2. Schwann Cells and Nerve Injury
Neurons and glial cells represent the major cell types constituting the nervous system. Although in the past, glia was considered only a passive component in brain functionality, today this opinion has completely changed. Based on various evidence, it has been demonstrated that glial cells actively regulate neuronal properties and activities [
4]. On the base of their morphological, biological, and functional characteristics, glial cells are classified into oligodendrocytes, microglia, ependimocytes, and astrocytes, located in the CNS, and Schwann and satellite glial cells, sited in the PNS.
Schwann cells, originating from the neural crest, are the most abundant glial cells of the PNS. It is not possible to consider these cells solely as passive insulators of axons, but rather they are co-stars in the regulation of neuronal biology by providing metabolic support and regulating the response to tissue injury [
5,
6,
7]. Schwann cells can be classified into Remak cells, or non-myelin Schwann cells, and myelin Schwann cells. Remak cells envelop the small diameter axons, including those of the autonomic and sensory nervous systems. These cells ensure proper development of the PNS and play an important role in regeneration after injury. In fact, the absence of myelin confers to Remak cells the ability to stimulate axonal plasticity, growth, and sprouting. Moreover, these cells modulate pain sensitivity in peripheral sensory neuropathies. In accord, it has been demonstrated that even in absence of injury, alterations between axon-Remak cells interactions are followed by neuropathic pain [
2,
8]. Meanwhile, Schwann cells wrap larger axons of sensory and motor neurons [
2,
9]. Myelinating Schwann cells are radially and longitudinally polarized cells surrounding the axon and delimiting nodal, paranodal, juxtaparanodal, and internodal compartments [
10]. Among these, the internodal compartment is the largest domain representing 99% of the myelinating Schwann cell length. Compared to Remak cells, these cells form a myelin sheath wrapping around the axon several times.
Myelin is characterized by high lipid content (70%) enriched with glycosphingolipids, saturated long-chain fatty acids, and cholesterol, the latter involved in the assembly of myelin sheath. Synthesis, maintenance, and structure of the myelin sheath is also regulated by several molecules including myelin protein zero (MPZ), pro-myelin transcription factor Egr2 (Krox20), maltose-binding protein (MBP), and myelin associated glycoprotein (MAG). MPZ is a transmembrane adhesion molecule of the immunoglobulin gene superfamily, needing cholesterol for proper endoplasmic reticulum (ER) export. Krox20 controls a set of genes required for the completion of nerve myelination [
13]. MBP is a peripheral membrane protein promoting myelin membrane stacking [
14], whereas MAG is a membrane associated protein supporting myelin-axon stability and regulating the axon cytoskeleton [
15,
16].
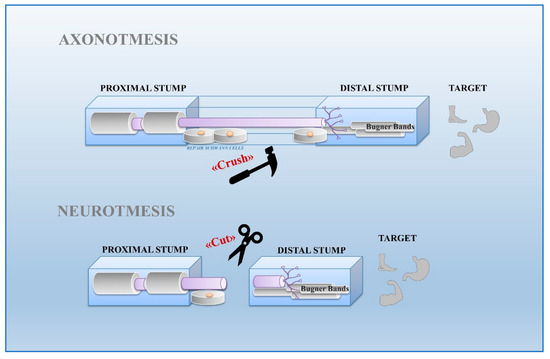
Schwann cells exhibit great plasticity, a peculiar feature allowing them to actively adapt and convert into cells sustaining regeneration following nerve injury [
17]. Nerve injuries can be classified by severity into three broad grades: neurapraxia, axonotmesis, and neurotmesis [
18] (
Figure 1). Neurapraxia is the mildest type of injury which does not imply loss of nerve continuity. It occurs after nerve compression or blocked blood flow to the affected nerves. It is characterized by a temporary failure to conduct signals due to a local ion-induced conduction block at the injury site or alterations in the myelin structure [
19].
Figure 1. Nerve regeneration in axonotmesis and neurotmesis. Axonotmesis consists in destruction of axons by nerve crush. Axons are severed but remain within intact tubes. Schwann cells of the bridge accompany axon regeneration to the Bungner band in the distal stump towards the target organ. Neurotmesis is characterized by the destruction of axons following a cut lesion. Schwann cells of proximal stump drive the regenerating fibers towards the Bungner band of the distal stump.
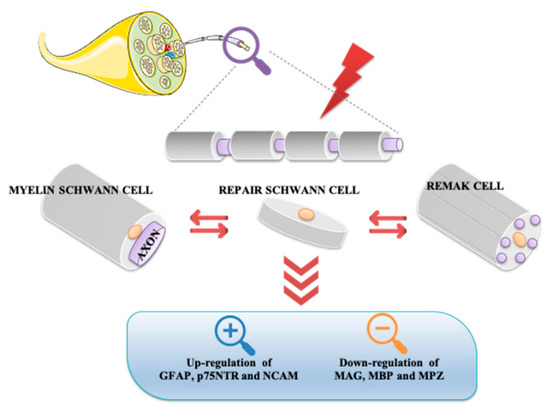
Remak and myelin Schwann cells are converted into repair Schwann cells both in axonotmesis and neurotmesis, as demonstrated by reversal in myelin differentiation markers expression in response to injury. In this event, molecules characterizing the immature Schwann cell stage, such as glial fibrillary acidic protein (GFAP), neural cell adhesion molecule (NCAM), and p75 neurotrophin receptor (p75NTR), are up regulated. On the contrary, key myelin forming molecules, including MAG, MBP, and MPZ, are downregulated (Figure 2).
Figure 2. Conversion of myelin Schwann and non-myelin Remak cells into repair Schwann cells after nerve injury. Either in axonotmesis or neurotmesis, repair Schwann cells show up-regulation of GFAP, NCAM, and p75NTR and down-regulation of some myelin genes. GFAP, glial fibrillary acidic protein; MAG, myelin associated glycoprotein; MBP, maltose-binding protein; MPZ, myelin protein zero; NCAM, neural cell adhesion molecule; p75NTR, p75 neurotrophin receptor.
During peripheral nerve regeneration, many macrophages infiltrate into nerve damaged area [
26]. The Schwann cells are involved in their recruitment through the release of cytokines including tumor necrosis factor (TNF)-a, interleukin (IL)-1a, IL-1b, and monocyte chemotactic protein 1 (MCP-1) from the distal stump [
27].
Moreover, Schwann cells promote the survival and axonal elongation of injured neurons by releasing several neurotrophic factors including glial cell line-derived neurotrophic factor (GDNF), brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT3), nerve growth factor (NGF), vascular endothelial growth factor (VEGF), artemin, and pituitary adenylyl cyclase-activating peptide (PACAP) [
29,
30,
31,
32].
3. Effect of PACAP on Schwann Cells during Nerve Injury
In response to peripheral nerve injury, the level of PACAP significantly increases both in sensory and motor neurons [
83,
84,
85]. In particular, in the cultured rat vagus nerve, it is up regulated and detectable up to one month after nerve damage, corresponding to the period of axon regeneration [
86]. In the site of damaged vagus nerve, PACAP up-regulation induces the stimulation of cells surrounding the regenerating fibers including Schwann cells [
86]. Moreover, Armstrong et al. [
87], showed that axon regeneration following injury is strongly reduced in PACAP knockout animals.
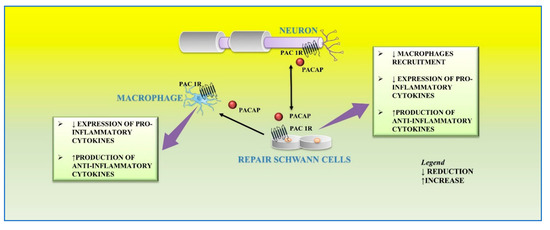
Axons of lesioned neurons release PACAP in the surrounding area, which binds to its receptors localized on cultured primary rat Schwann cells and macrophages. This peptide plays different functions on the distal stump related to different phases of regeneration. After peripheral nerve injury, a large number of macrophages infiltrates into the distal nerve stump area to clear axonal and myelin debris. This event is further sustained by the release of proinflammatory cytokines from resident macrophages that promote the recruitment of other macrophages to the distal stump [
88]. Moreover, Schwann cells are involved in their recruitment through the release of cytokines, such as TNF-a, interleukin (IL)-1a, IL-1b, and monocyte chemotactic protein 1 (MCP-1) from the distal stump [
27]. Therefore, this inflammatory vicious cycle must be regulated in order to avoid excessive inflammation and further tissue damage. In the early step of peripheral nerve injury, PACAP plays a regulatory effect by balancing pro-inflammatory cytokine production from Schwann cells and preventing excessive macrophage recruitment. The paracrine role of PACAP in neuronal survival and axon outgrowth during peripheral nerve regeneration is related to the up-regulation of its receptors. In accord, high expression levels of PAC1, VPAC1, and VPAC2 receptors have been demonstrated in both Schwann cells and macrophages within the distal stump after mouse sciatic nerve injury [
32]. Stimulation of PACAP receptors may rapidly activate MAPK/ERK or PI3K/Akt pathways, both involved in maintenance of myelinated peripheral nerves and proliferation of Schwann cells after injury [
89,
90].
Moreover, the exposure of the RT4-D6P2T Schwann cell line to an inflammatory stimulus, such as lipopolysaccharide (LPS), significantly increased not only the levels of pro-inflammatory cytokines such as IL-6, IL-18, and TNF but also PACAP release [
91].
In the later step of regeneration, PACAP, released from neurons as well as Schwann cells, down-regulates the expression of pro-inflammatory cytokines and up-regulates the production of anti-inflammatory cytokines from macrophages by favoring the macrophage stage transition in order to end the inflammatory response in the distal nerve stump. In accord, it has been found that PACAP increases the expression of anti-inflammatory cytokines, such as IL-4, IL-10, and IL-13, in the distal nerve explant [
32] (
Figure 3).
Figure 3. Autocrine and/or paracrine action of PACAP on inflammatory response and myelinization of peripheral nerve following injury.
At the end of peripheral nerve regeneration, PACAP induces re-myelination promoted by Schwann cells. Many studies have demonstrated that the peptide binding to PAC1R is involved in the myelination processes of the CNS by promoting the development of oligodendrocytes from oligodendroglia progenitor cells [
76,
92].
Furthermore, PACAP also induces myelination processes in the PNS [
87]. PACAP may act as a predisposing factor able to switch in Schwann cells those intracellular events that anticipate the assembly of new myelin, by increasing the expression of genes encoding for its essential components [
93]. In fact, exogenous treatment with the peptide significantly increases the expression of the major protein components of myelin, such as MBP, MAG, and MPZ, via stimulation of the PI3K/Akt signaling pathway. This signaling cascade is linked to the differentiation and survival process of Schwann cells [
79,
94,
95,
96]. In the latter, PACAP also acts as a potent inducer of tissue plasminogen activator (tPA) through binding to PAC1R activating the Akt/CREB signaling pathway in a rat schwannoma cell line [
79]. In the context of nerve injuries, the release of tPA from Schwann cells of mice sciatic nerve promotes axonal regeneration and re-myelination [
97].
The effects of PACAP are not limited to the aforementioned biological events. In fact, PACAP also plays a trophic role in Schwann cells by promoting their survival. In accord, it has been previously demonstrated that treatment with PACAP prevents apoptotic cell death in serum deprived RT4 schwannoma cell line [
78].
By analyzing the skin transcriptional profile of 60 patients undergoing carpal tunnel surgery, 31 differentially expressed genes were identified following decompression. Among these, the ADCYAP1 gene, encoding for PACAP, was the main upregulated gene and linked to the recovery of intraepidermal nerve fibers. Moreover, the peptide significantly promoted dose-dependent axon outgrowth in human induced pluripotent stem-cell-derived sensory neurons [
98].
As highlighted by the aforementioned literature, different experimental approaches have been used to study the role of Schwann cells on peripheral nerve injury and regeneration, including cell line-based models, primary cell-based models, and ex vivo explants [
99]. Although the use of cell lines shows doubtless ethical advantages, it has many limitations. In fact, biological properties of cell culture in vitro are far distant from those of the corresponding cells in vivo. Moreover, some cell lines (e.g., RT4-D6P2T) are derived from tumors. These cells offer the advantage of being free of cellular senescence; however, compared to primary Schwann cells, they do not express some genes supporting nerve repair and remyelination after acute nerve injury [
100].