Phosphorus resources, both in phosphate rocks and in the soil, are limited. However, effective food production is not possible without the use of P fertilizers. Recognizing and eliminating or at least ameliorating factors (hot spots) that interfere with the uptake and use of phosphorus (P) by crop plants is of key importance for effective use of both P and nitrogen (N) on the farm. Plants have developed many adaptation mechanisms to their environment, i.e., soil low in available phosphorus. The most important ones include the secretion of organic compounds into the rhizosphere and the association of plant roots with microorganisms. A classic example is mycorrhiza. These mechanisms can be used by the farmer to sequentially select plants in the crop rotation. The uptake of inorganic P (Pi) by plants from the soil is reduced by environmental (temperature and water) and soil factors (low content of available phosphorus, soil acidity, soil compaction). These factors are responsible for the growth and size of the root system. Mitigating these negative effects improves the efficiency of phosphorus uptake from the soil. The second group of critical factors, limiting both root growth and availability of phosphorus, can be effectively controlled using simple measures (for example, lime).
- crop plants
- farm
- phosphorus uptake
- phosphorus
- phosphorus sources
1. Introduction
2. Mechanisms of Phosphorus Uptake
- (1)
-
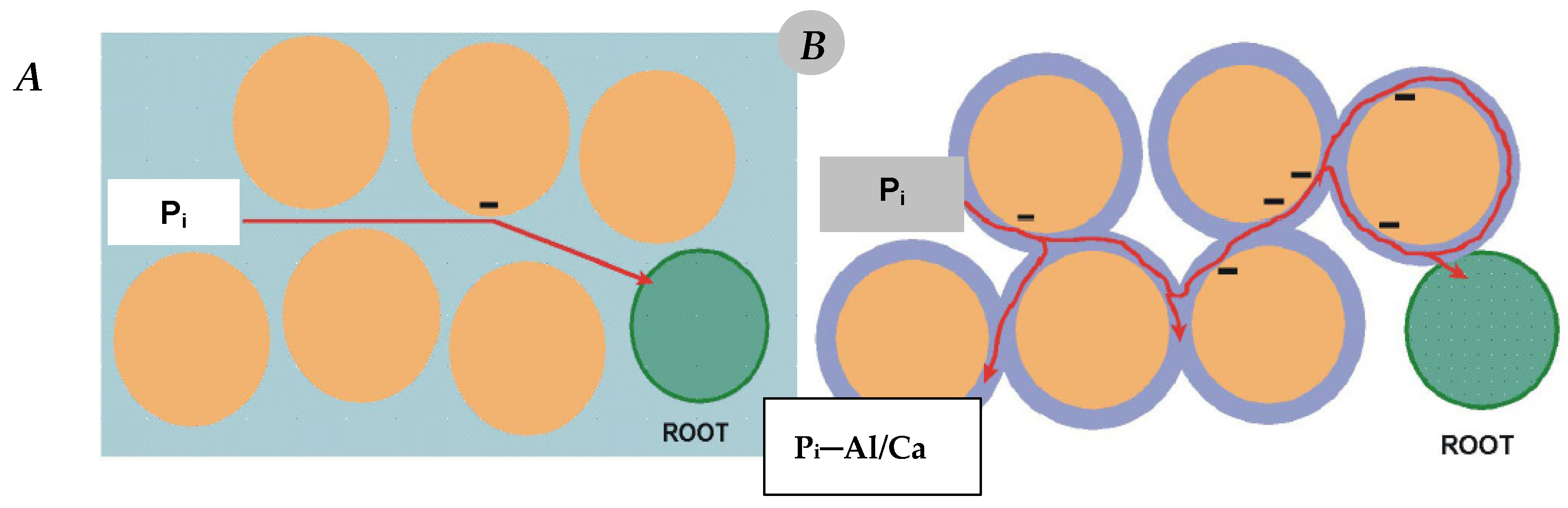
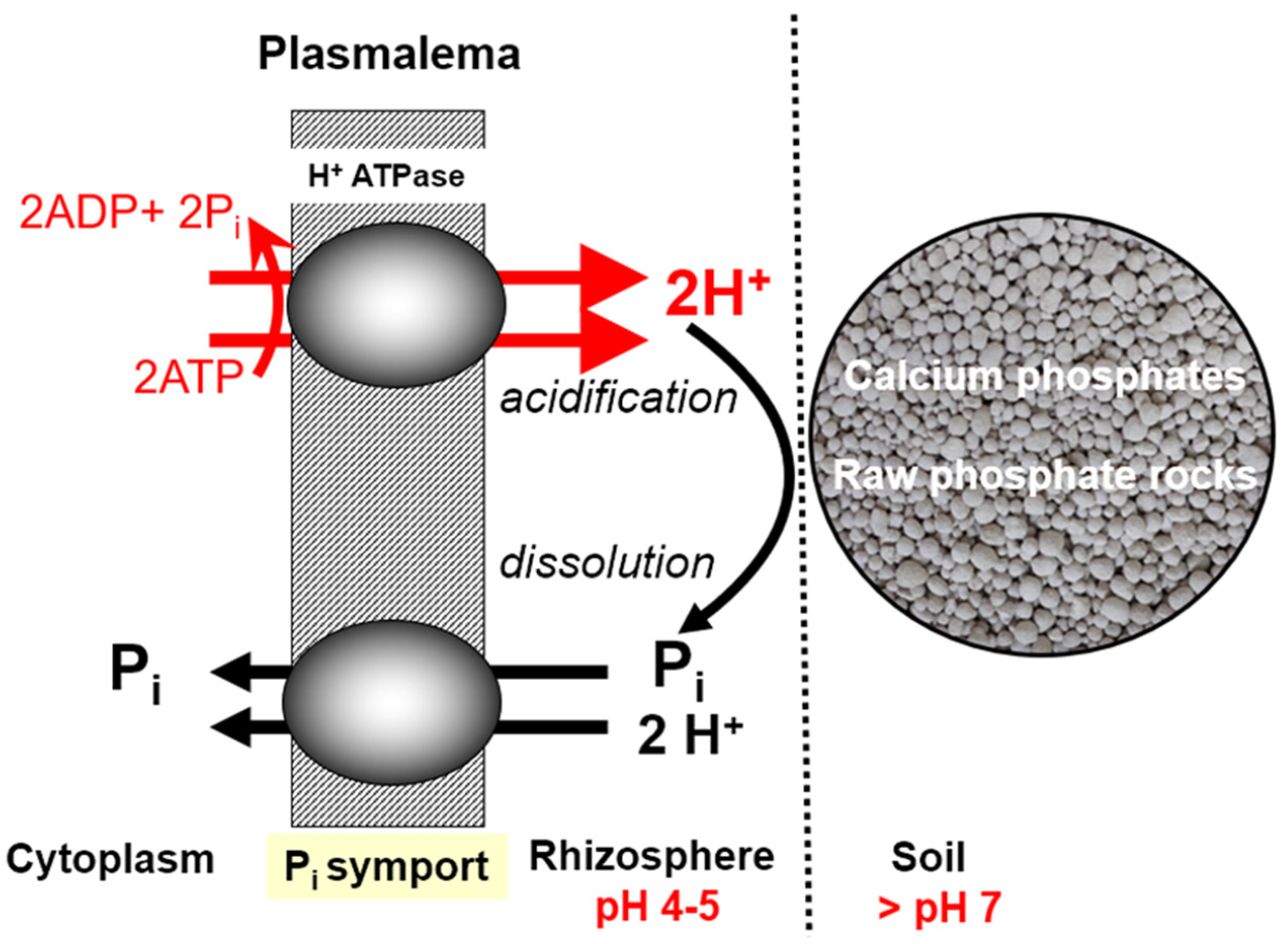
Passive—flow of Pi anions in the soil solution towards the plant root surface;
- (2)
-
Active—transfer of the Pi ions across the plasmalemma of the cortical root cell.
- (1)
-
Pi concentration in the soil solution;
- (2)
-
Root absorption surface;
- (3)
-
Transport rate of Pi ions from the soil solution to the root surface;
- (4)
-
The rate of Pi ions’ incorporation into the plant’s metabolic processes.
3. Response Strategies of Crop Plants to Pi Deficiency
| PSM Isolates | Organic Acids | References |
|---|---|---|
| Pseudomonas | Citric acid, succinic acid, fumaric acid, gluconic acid, 2-ketogluconic acids | [42][43] |
| Bacillus sp. | Citric acid, malic acid, succinic acid, fumaric acid, tartaric acid, gluconic acid | [42] |
| Proteus sp. | Citric acid, succinic acid, fumaric acid, gluconic acid | [42] |
| Azospirillum sp. | Citric acid, succinic acid, fumaric acid, gluconic acid | [42] |
| Aspergillus | Citric acid, gluconic acid, oxalic acid, succinic acid, malic acid, glycolic acid | [44] |
| Penicillium sp. | Gluconic acid, glycolic acid, succinic acid, malic acid, oxalic acid, citric acid | [44] |
| Erwinia herbicola | Gluconic acid, 2-ketogluconic acid | [43] |
4. Environmental Factors Controlling Phosphorus Uptake by Crop Plants
4.1. Temperature
4.2. Water—Drought
- (1)
-
Cells: (i) water photolysis—water acts as a nutrient; (ii) a component of spatial structures of organic compounds, including proteins, carbohydrates, and fats; (iii) a component of the osmotic sap of cells;
- (2)
-
Tissues and organs: (i) connects cells to form tissues and then organs; (ii) determines the turgor of conducting cells (xylem); (iii) a critical component of assimilates transported in the phloem;
- (3)
-
Plant: controls (i) the optimum level of temperature through transpiration of water and thus the rate of metabolic processes, (ii) the movement of stomata during the circadian cycle, and (iii) CO2 uptake from the atmosphere.
This entry is adapted from the peer-reviewed paper 10.3390/agronomy14010200
References
- Van Dijk, M.; Morley, T.; Rau, M.L.; Saghai, Y. A meta-analysis of projected global food demand and population at risk of hunger for the period 2010–2050. Nat. Food 2021, 2, 494–501.
- Year Book for 2009; FAO: Rome, Italy, 2009.
- Alexander, P.; Brown, C.; Ameth, A.; Finnigan, J. Human appropriation for land and food: The role of diet. Glob. Environ. Chang. 2016, 41, 88–98.
- Berners-Lee, M.; Kennelly, C.; Watson, R.; Hewitt, C.N. Current global food production is sufficient to meet human nutritional needs in 2050 provided there is radical societal adaptation. Elem. Sci. Anth. 2018, 5, 52.
- Gomiero, T. Soil degradation, land scarcity and food security: Reviewing a complex challenge. Sustainability 2016, 8, 281.
- Roy, E.D.; Richards, P.D.; Martinelli, L.A.; Coletta, L.D.; Machado Lins, S.R.; Vazquez, F.F.; Willing, E.; Spera, S.A.; Van Wey, L.L.K.; Porder, S. The phosphorus cost of agricultural intensification in the tropics. Nat. Plants 2016, 2, 16043.
- Röös, E.; Bajželj, B.; Smith, P.; Patel, M.; Little, D.; Garnett, T. Greedy or needy? Land use and climate impacts of food in 2050 under different livestock futures. Glob. Environ. Chang. 2017, 47, 1–12.
- Stewart, W.M.; Roberts, T.L. Food security and the role of fertilizers in supporting it. Procedia Eng. 2012, 46, 76–82.
- Izumi, T.; Sakai, T. The global data set of historical yield of major crop 1981–2016. Sci. Data 2020, 7, 97.
- Ray, D.K.; Mueller, N.D.; West, P.C.; Foley, J.A. Yield trends are insufficient to double global crop production by 2050. PLoS ONE 2013, 8, e66428.
- Cordell, D.; White, S. Life’s Bottleneck: Sustaining the world’s phosphorus for a food secure future. Ann. Rev. Environ. Resour. 2014, 39, 161–168.
- Marschner, P. Marchner’s Mineral Nutrition of Higher Plants, 3rd ed.; Elsevier Ltd.: London, UK, 2012; p. 649.
- Pereira, A.M.; Coimbra, S. Advances in plant reproduction: From gametes to seeds. J. Exp. Bot. 2019, 15, 2933–2936.
- Grzebisz, W.; Diatta, J.; Barłóg, P.; Biber, M.; Potarzycki, J.; Łukowiak, R.; Przygocka-Cyna, K.; Szczepaniak, W. Soil Fertility Clock—Crop Rotation as a Paradigm in Nitrogen Fertilizer Productivity Control. Plants 2022, 11, 2841.
- Nicholls, J.W.; Chin, J.P.; Williams, T.A.; Lenton, T.M.; O’Flaherty McGrath, J.W. On the potential roles of phosphorus in the early evolution of energy metabolism. Front. Microbiol. 2023, 14, 1239189.
- Lima Viana, J.; Moretti de Souza, J.L.; Hoshide, A.K.; de Oliveira, R.A.; de Abreu, D.C.; da Silva, W.M. Estimating sugarcane yield in subtropical climate using climatic variable and soil water storage. Sustainability 2023, 15, 4360.
- Tanveer, A.; Ikram, R.M.; Ali, H.H. Crop rotation: Principles and practices. In Agronomic Crops; Hasanuzzaman, M., Ed.; Springer: Singapore, 2019; pp. 1–13.
- MacDonald, G.K.; Bennett, E.M.; Potter, P.A.; Ramankutty, N. Agronomic phosphorus imbalances across the world’s croplands. Proc. Natl. Acad. Sci. USA 2011, 108, 3086–3091.
- Łukowiak, R.; Grzebisz, W.; Sassenrath, G.F. New insights into phosphorus management in—A crop rotation approach. Sci. Tot. Environ. 2016, 542, 1062–1077.
- Lambers, H. Phosphorus acquisition and utilization in plants. Ann. Rev. Plant Biol. 2022, 73, 17–42.
- Wang, Y.; Wang, F.; Lu, H.; Mao, C. Phosphate uptake and transport in plants: An elaborate regulatory system. Plant Cell Physiol. 2021, 62, 564–572.
- Barber, S.A. Soil Nutrient Bioavailability: A Mechanistic Approach, 2nd ed.; Wiley and Sons: New York, NY, USA, 1995; p. 432.
- McKay Fletecher, D.M.; Ruiz, S.; Dias, T.; Petroselli, C.; Roose, T. Linking root structure to functionality: The impact of root system architecture to citrate enhanced phosphate uptake. New Phytol. 2020, 227, 376–391.
- Giehl, R.F.H.; Wiren, N. Root nutrient foraging. Plant Physiol. 2014, 166, 509–517.
- Lynch, J.L. Root phenotypes for improved capture: An underexploited opportunity for global agriculture. New Phytol. 2019, 223, 548–564.
- Nielsen, N.E. Nutrients: Bioavailability and plant uptake. In Managing Global Resources and Universal Processes; Fath, B.D., Jorgensen, S.E., Cole, M., Eds.; CRC Press: London, UK, 2020; pp. 501–509.
- De Sousa Nunes, R.; Gones de Sousa, D.M.; Goedert, W.J.; Zancanaro de Oliveira, L.E.; Pinheiro, T.D. Vrops; yield and roots response to soil phosphorus distribution resulting from long-term soil and phosphate fertilization management strategies. Front. Agron. 2021, 3, 757100.
- McKay, A.D.; Barber, S.A. Effect of soil moisture and phosphate level on root hair growth on corn roots. Plant Soil 1985, 86, 321–331.
- Ciampitti, I.A.; Camberato, J.J.; Murrel, S.T.; Vyn, T.J. Maize nutrient accumulation and partitioning in response to plant density and nitrogen rate. I. Macronutrients. Agron. J. 2013, 10593, 7830.
- Julia, C.; Wissuva, M.; Kretzschmar, T.; Jeong, K.; Rose, T. Phosphorus uptake, partitioning and redistribution during grain filling in rice. An. Bot. 2016, 118, 1151–1162.
- Subedi, K.; Ma, B. Nitrogen uptake and partitioning in stay-green and leafy maize hybrids. Crops Sci. 2005, 45, 740–747.
- Guan, P. Dancing with hormones: A current perspective of nitrate signaling and regulation in Arabidopsis. Front. Plant Sci. 2017, 8, 1697.
- Luo, L.; Zhang, Y.; Xu, G. How does nitrogen shape plant architecture. J. Exp. Bot. 2020, 71, 4415–4427.
- Cordell, D.; Drangert, J.-O.; White, S. The story pf phosphorus global food security and food for thought. Glob. Environ. Chang. 2009, 19, 292–305.
- Grzebisz, W.; Szczepaniak, W.; Barłóg, P.; Przygocka-Cyna, K.; Potarzycki, J. Phosphorus sources for winter oilseed rape (Brassica napus L.) during reproductive growth—Magnesium sulfate management impact on P use efficiency. Arch. Agron. Soil Sci. 2018, 64, 1646–1662.
- Hasan, M.M.; Hasan, M.M.; Texeira da Silva, J.A.; Li, X. Regulation of phosphorus uptake and utilization: Transitioning from current knowledge to practical strategies. Cell. Mol. Biol. Lett. 2016, 21, 7.
- Maathuis, F.J.M. Physiological functions of mineral macronutrients. Curr. Opin. Plant Biol. 2009, 12, 250–258.
- Bhadouria, J.; Giri, J. Purple acid phosphatases: Roles in phosphate utilization and new emerging functions. Plant Cell Rep. 2022, 41, 33–51.
- Ciereszko, I.; Żebrowska, E.; Ruminowicz, M. Acid phosphatases and growth of barley (Hordeum vulgare L.) cultivars under diverse phosphorus nutrition. Acta Physiol. Plant. 2011, 33, 2355–2368.
- Shen, Q.; Wen, Z.; Dong, Y.; Li, H.; Miao, Y.; Shin, J. The responses of root morphology and phosphorus-mobilizing exudations in wheat to increasing shoot phosphorus concentration. AoB Plants 2018, 10, ply054.
- López-Arrendondo, D.L.; Leyva-González, M.A.; González-Morales, S.I.; López-Bucio, J.; Herrera-Estrella, L. Phosphate nutrition: Improving low-phosphate tolerance. Ann. Rev. Plant Biol. 2014, 65, 95–123.
- Selvi, K.B.; Paul, J.J.A.; Vijaya, V.; Saraswathi, K. Analyzing the efficacy of phosphate solubilizing microorganisms by enrichment culture techniques. Bioch. Mol. Biol. J. 2017, 3, 1–7.
- Kumar, A.; Kumar, A.; Patel, H. Role of microbes in phosphorus availability and acquisition by plants. Inter. J. Curr. Microb. Appl. Sci. 2018, 7, 1344–1347.
- Sane, S.A.; Mehta, S.K. Isolation and evaluation of rock phosphate solubilizing fungi as potential biofertilizer. J. Fertil Pestic. 2015, 6, 156–160.
- Garg, N.; Chandel, S. Arbuscular mycorrhizal networks: Process and functions: A review. Agron. Sustain. Dev. 2010, 30, 581–599.
- Vance, C.P. Plants without arbuscular mycorrhizae. In The Ecophysiology of Plant-Phosphorus Interactions; White, P.J., Hammond, J.P., Eds.; Springer Science + Business Media BV: Berlin/Heidelberg, Germany, 2008; pp. 117–142.
- Sharma, S.B.; Sayyed, R.Z.; Trivedi, M.H.; Gobi, T.A. Phosphate solubilizing microbes: Sustainable approach for managing phosphorus deficiency in agricultural soils. SpringerPlus 2013, 2, 587–600.
- Spatafora, J.W.; Chang, Y.; Benny, G.L.; Lazarus, K.; Smith, M.E.; Berbee, M.L.; Stajich, J.E. A phylum-level phylogenetic classification of zygomycete fungi based on genome-scale data. Mycologia 2016, 108, 1028–1046.
- Davison, J.; Moora, M.; Öpik, M.; Adholeya, A.; Ainsaar, L.; Bâ, A.; Zobel, M. Global assessment of arbuscular mycorrhizal fungus diversity reveals very low endemism. Science 2015, 349, 970–973.
- Giovannini, L.; Palla, M.; Agnolucci, M.; Avio, L.; Sbrana, C.; Turrini, A.; Giovannetti, M. Arbuscular mycorrhizal fungi and associated microbiota as plant biostimulants: Research strategies for the selection of the best performing inoculate. Agronomy 2020, 10, 106.
- Baum, C.; El-Tohamy, W.; Gruda, N. Increasing the productivity and product quality of vegetable crops using arbuscular mycorrhizal fungi: A review. Sci. Hort. 2015, 187, 131–141.
- Augé, R.M.; Toler, H.D.; Saxton, A.M. Arbuscular mycorrhizal symbiosis alters stomatal conductance of host plants more under drought than under amply watered conditions: A meta-analysis. Mycorrhiza 2015, 25, 13–24.
- Etesami, H.; Jeong, B.R.; Glick, B.R. Contribution of arbuscular mycorrhizal fungi, phosphate–solubilizing bacteria, and silicon to P uptake by plant. Front. Plant Sci. 2021, 12, 699618.
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ashraf, M.; Zhang, L. Role of arbuscular mycorrhizal fungi in plant growth regulation: Implications in abiotic stress tolerance. Front. Plant Sci. 2019, 10, 1068.
- Thirkell, T.J.; Cameron, D.D.; Hodge, A. Resolving the ‘nitrogen paradox’ of arbuscular mycorrhizas: Fertilization with organic matter brings considerable benefits for plant nutrition and growth. Plant Cell Environ. 2016, 39, 1683–1690.
- Péret, B.; Clément, M.; Nussaume, L.; Desnos, T. Root developmental adaptation to phosphate starvation: Better safe than sorry. Trends Plant Sci. 2011, 16, 442–450.
- Shane, M.W.; Lambers, H. Cluster roots: A curiosity in context. Plant Soil 2005, 27, 101–125.
- Abdolzadeh, A.; Wang, X.; Veneklaas, E.J.; Lambers, H. Effects of phosphorus supply on growth, phosphate concentration and cluster-root formation in three Lupinus species. Ann. Bot. 2010, 105, 365–374.
- Liu, D. Root developmental responses to phosphorus nutrition. J. Integr. Plant Biol. 2021, 63, 1065–1090.
- White, C.; Sylvester-Bradley, R.; Berry, P.M. Root length densities of UK wheat and oilseed rape crops with implications for water capture and yield. J. Exp. Bot. 2015, 66, 2293–2303.
- Remus, R.; Pandey, D.; Lüttschwager, D. What regulates the rhizodeposition of winter oilseed rape during growth. Plant Soil 2022, 478, 283–310.
- Duan, X.; Jin, K.; Ding, G.; Wang, C.; Cai, H.; Wang, S.; White, P.J.; Xu, F.; Shi, L. The impact of different morphological and biochemical traits on phosphorus acquisition and seed yield of Brassica napus. Field Crops Res. 2020, 258, 107960.
- Ziadi, N.; Whalen, J.K.; Messiga, A.J.; Morel, C. Assessment and modelling of soil available phosphorus in sustainable cropping systems. Adv. Agron. 2013, 122, 85–126.
- McMichael, B.L.; Burke, J.J. Soil temperature and root growth. HortScience 1998, 33, 947–951.
- Kaspar, T.C.; Bland, W.L. Soil temperature and root growth. Soil Sci. 1992, 154, 290–299.
- Fonseca de Lima, C.F.; Klein-Vehn, J.; De Smet, I.; Feraru, E. Getting to the root belowground high temperature responses in plants. J. Exp. Bot. 2021, 72, 7404–7413.
- Hatfield, J.I.; Prueger, J.H. Temperature extremes: Effect on plant growth and development. Weather Clim. Extrem. 2015, 10, 4–10.
- Xu, H.; Hassan, M.A.; Sun, D.; Wu, Z.; Jiang, G.; Liu, B.; Ni, Q.; Yang, W.; Fang, H.; Li, J.; et al. Effects of low temperature stress on source-sink organs in wheat and phosphorus mitigation strategies. Front. Plant Sci. 2022, 13, 807844.
- Tiwari, M.; Kumar, R.; Subramanian, S.; Doherty, C.J.; Krishna Jagadish, S.V. Auxin-cytokinin interplay shapes root functionality under low-temperatures stress. Trends Plant Sci. 2023, 28, 447–459.
- Zhu, J.; Zhang, K.-X.; Wang, W.-S.; Gong, W.; Liu, W.-C.; Chen, H.-G.; Xu, H.-H.; Lu, Y.-T. Low temperature inhibits root growth by reducing auxin accumulation via ARR1/12. Plant Cell Physiol. 2015, 56, 727–736.
- Sadok, W.; Krishna Jagadish, S.V. The hidden cost of nighttime warming on yields. Trends Plant Sci. 2020, 25, 644–651.
- Calleja-Cabrera, J.; Boter, M.; Onate-Sanchez, L.; Pernas, M. Root growth adaptation to climate change in crops. Front. Plant Sci. 2020, 11, 544.
- Ben-Noach, I.; Friedman, S.P. Review and evaluation of root respiration and of natural and agricultural processes of soil respiration. Vadose Zone J. 2018, 17, 1–47.
- Mittler, R.; Finka, A.; Goloubinoff, P. How do plants feel the heat? Trends Biochem. Sci. 2012, 37, 118–125.
- Singh, S.K.; Reddy, V.R.; Fleisher, D.H.; Timlin, D.J. Phosphorus nutrition affects temperature response of soybean growth and canopy photosynthesis. Front. Plant Sci. 2018, 9, 1116.
- Rabbinge, R. The ecological background of food production. In Crop Production and Sustainable Agriculture; Rabbinge, R., Ed.; John Wiley and Sons: New York, NY, USA, 1993; pp. 2–29.
- Ievinsh, G. Water content of plant tissues: So simple that almost forgotten. Plants 2023, 12, 1238.
- Chavarria, G.; Pessoa des Santos, H. Plant water relations: Absorption, transport and control mechanisms. In Advances in Selected Plant Physiology Aspects; Montanaro, G., Ed.; Intech Europe: Rijeka, Croatia, 2012; pp. 105–133.
- Steudle, E. Water uptake by roots: Effects of water deficit. J. Exp. Bot. 2000, 51, 1531–1542.
- Kou, X.; Han, W.; Kang, J. Responses of root system architecture to water stress at multiple levels: A meta-analysis of trials under controlled conditions. Front. Plant Sci. 2022, 13, 1085409.
- Smucker, A.J.M.; Aiken, R.M. Dynamic root responses to water deficits. Soil Sci. 1992, 154, 281–289.
- Fromm, H. Root plasticity in the pursuit of water. Plants 2019, 8, 236.
- Cai, Q.; Zhang, Y.; Sun, Z.; Zheng, J.; Bai, W.; Zhang, Y.; Liu, Y.; Feng, L.; Feng, C.; Zhang, Z.; et al. Morphological plasticity of root growth under mild water stress increases water use efficiency without reducing yield on maize. Biogeosciences 2017, 14, 3851–3858.
- Fry, E.L.; Evans, A.L.; Sturrock, C.J.; Bullock, J.M.; Bardgett, R.D. Root architecture governs plasticity in response to drought. Plant Soil 2018, 433, 189–200.
- Luo, Y.-Z.; Li, G.; Yan, G.; Liu, H.; Turner, N.C. Morphological features and biomass partitioning of lucerne plants (Medicago sativa L.) subjected to water stress. Agronomy 2020, 10, 322.
- Bodner, G.; Nakhforoosh, A.; Kaul, H.P. Management of crop water under drought: A review. Agron. Sustain. Dev. 2015, 35, 401–442.
- Al-Kaisi, M.; Broner, I. Crop Water Use and Growth Stages; CSA, No. 4.715; Colorado State University Extension: Fort Collins, CO, USA, 2009; p. 4.
- Passioura, J. Increasing crop productivity when water is scarce—From breeding to field management. Agric. Water Manag. 2006, 80, 176–196.
- Kang, S.; Gu, B.; Du, T.; Zhang, J. Crop coefficient and ration of transpiration to evapotranspiration of winter wheat and maize in a semi-humid region. Agric. Water Manag. 2003, 59, 239–254.
- Sah, R.P.; Chacraborty, M.; Prasad, K.; Pandit, M.; Tudu, V.K.; Chakravarty, M.K.; Narayan, S.C.; Rana, M.; Moharana, D. Impact of water deficit stress in maize: Phenology and yield components. Sci. Rep. 2020, 10, 2944.
- He, M.; Dijkstra, F.A. Drought effect on plant nitrogen and phosphorus: A meta-analysis. New Phytol. 2014, 204, 924–931.
- Rouphael, Y.; Cardarelli, M.; Schwarz, D.; Franken, P.; Colla, G. Effects of drought on nutrient uptake and assimilation in vegetable crops. In Plant Responses to Drought Stress; Aroca, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 171–195.
- Rodriguez, D.; Goudriaan, J. Effects of phosphorus and drought stress an dry matter and phosphorus allocation in wheat. J. Plant Nutr. 1995, 189, 2501–2517.
- Dixon, M.; Simonne, E.; Obreza, T.; Liu, G. Crop response to low phosphorus bioavailability with focus on tomato. Agronomy 2020, 10, 617.
- Körner, C. Paradigm shift in plant growth control. Curr. Opin. Plant Biol. 2015, 25, 107–114.
- Najewski, A.; Madajska, K.; Skrzypek, A. Preliminary results of cultivar yield in post-registration experiments. In Winter Cereals; COBORU: Słupia Wielka, Poland, 2023; p. 18.
- Meki, M.N.; Osorio, J.M.; Steglich, E.M.; Kiniry, J.R. Drought-induced nitrogen and phosphorus carryover nutrients in corn/sybean rotations in the Upper Mississippi River Basin. Sustainability 2022, 14, 15108.
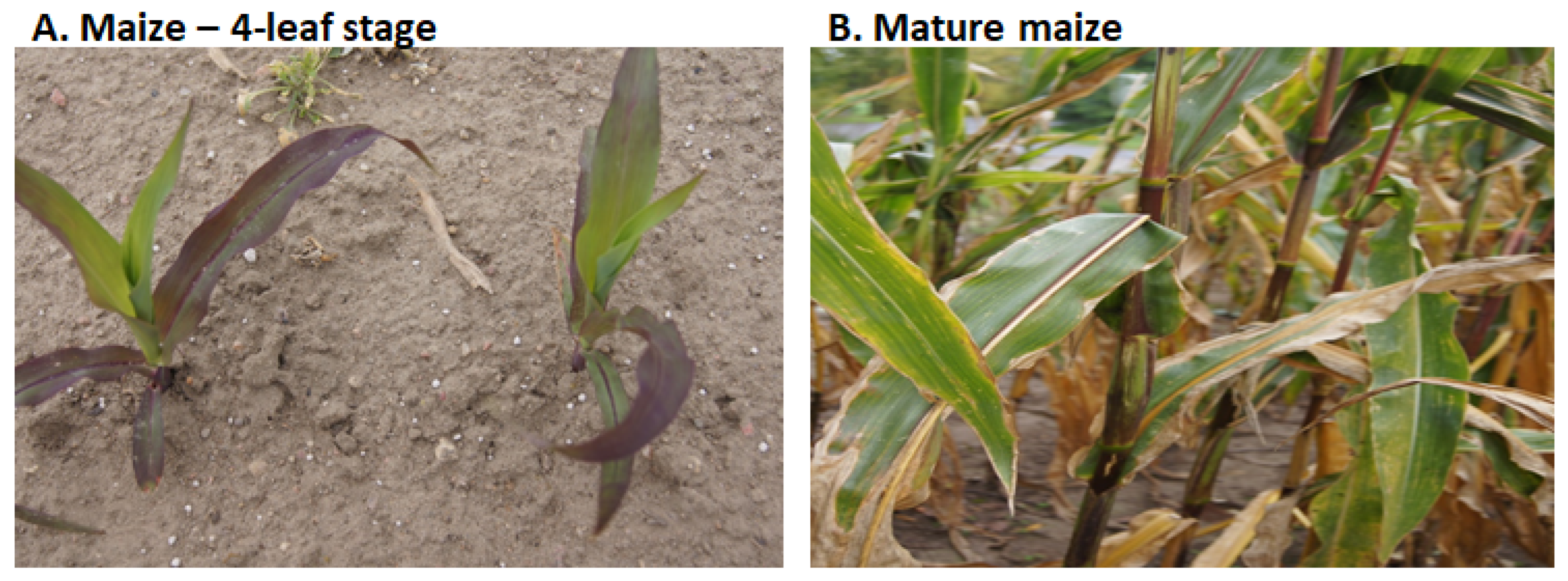
- Garcia-Oliveira, A.L.; Chander, S.; Barcelo, J.; Poschenrieder, C. Aluminum stress in plants. In Recent Advances in Plant Stress Physiology; Yadav, P., Kumar, S., Jain, V., Eds.; Daya Publishing House: New Delhi, India, 2016; pp. 265–282.
