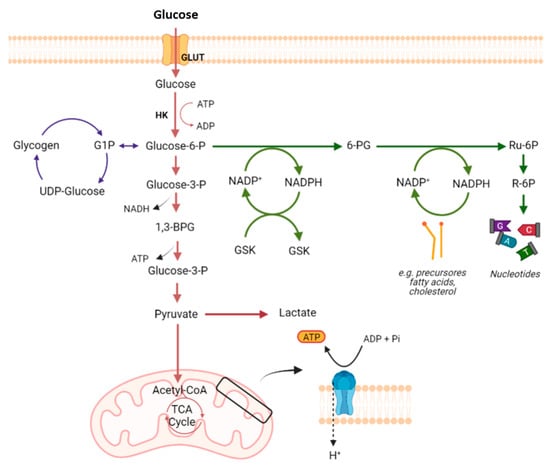
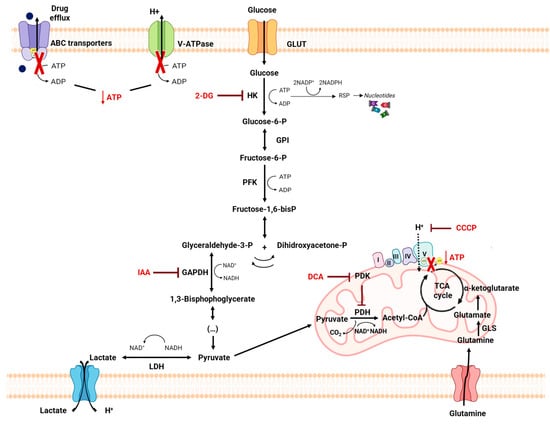
Amino acid metabolism can be also related to MDR phenotype, as it provides cancer cells with specific adaptive characteristics to neutralize the mechanism of action of the antitumor drugs to which they are exposed [
161]. In fact, amino acids play an important role both in most biosynthetic pathways, which are upregulated in cancer cells, and in maintaining the redox homeostasis balance [
113]. Among these, glutamine plays a crucial role in cancer metabolism and in drug resistance in cancer cells, since glutaminolysis supports the biosynthesis of many essential molecules [
105,
162] (
Figure 6). The importance of glutamine is also due to the fact that it is the amino acid with the largest carbon source for the TCA cycle. In the context of tumor cells, glutamine metabolism can provide essential building blocks for the excessive demands of both glycolysis and OXPHOS [
163].
4.4. Self-Delivery of Nanomedicine to Overcome Drug Resistance
Chemotherapy, radiation therapy and resection surgery remain the three “gold standard” anticancer therapies [
174]. Whether radiotherapy and surgery can be indicated for localized cancers, chemotherapy is considered the most appropriate treatment for most patients with metastasis and advanced cancer, as chemotherapy drugs can be widely distributed in the organism through the bloodstream [
175]. Nevertheless, the development of drug resistance and the low hydrosolubility of drugs are significant problems that restrict the clinical use of currently available chemotherapy drugs [
175]. Major chemotherapeutic agents include compounds like platinum complexes, DOX, vinca alkaloids, and taxanes, and primarily affect nucleic acids and protein synthesis, interfering with cell cycle and triggering apoptosis [
176,
177]. However, most of the standard agents approved for clinical use do not have the capacity to differentiate normal cells from cancer cells. This leads to serious side effects, especially in rapidly growing cells, as these drugs generally compromise mitosis. These cells include hair follicles, bone marrow cells and the gastrointestinal system, leading to hair loss, immune system failure, and infections, respectively [
178]. Thus, the decrease in the toxicity and side effects of the main chemotherapeutic agents is an urgent problem that needs to be overcome [
176]. To overcome this problem, various compounds, such as 3BP, DCA and 2DG, that interfere with metabolism, have been tested and demonstrated their ability to decrease tumor cell metabolism [
37]. However, there are disadvantages to a metabolism-based approach in cancer therapy, since the metabolic pathways required for cell survival are also present in normal cells. Thus, metabolism-based treatment can face the major hurdle of non-specific toxicity [
5]. To decrease their toxic side effects and increase antitumor efficacy, a number of drug delivery systems have been developed, such as albumin-bound PTX (Abraxane
®) or liposome-entrapped PTX and DOX, which have received clinical approval, as these formulations presented enhanced security but maintained their effectiveness [
175,
176,
179].
Nanotechnology-based cancer therapies aim to find new therapeutic methodologies correlated with disease mechanisms. The use of nanoparticles to encapsulate the drugs may increase the specificity of delivery to cancer cells and decrease the interaction with other non-cancer cells involved in tumor growth and spreading [
174]. Poly(lactic-
co-glycolic acid) (PLGA), a synthetic thermoplastic aliphatic biodegradable and biocompatible polyester, is widely studied and is one of the most characterized polymers [
184]. PLGA is degraded in non-toxic products (H
2O and CO
2) that are easily excreted [
176,
184]. Its polymeric NPs are degraded in vivo into lactate and glycolate. D-lactate is not metabolized prior to excretion and L-lactate is transformed into CO
2, which is eliminated by pulmonary excretion, or converted to pyruvate, which fuels the TCA cycle. Glycolate can be directly excreted by the kidneys or can be oxidized to glyoxylate, which is, in turn, further metabolized producing glycine, serine, and pyruvate. Subsequently, pyruvate can re-enter the TCA cycle and follow the OXPHOS pathway [
184]. The lactic acid (LA)/glycolic acid (GA) proportion is a good indicator not only when adjusting the degradation time, but also of the drug release rate [
184,
185]. Due to the absence of lateral methyl groups in GA, it has a higher hydrophilia, and thus, when higher amounts of GA are present, a higher degradation rate is observed [
184,
186].
Some PLGA polymers are FDA-approved materials and various PLGA NPs formulations have been clinically introduced, such as a formulation targeting advanced prostate cancer, ELIGARD
® [
178]. PLGA NPs were also shown to be effective in increasing the accumulation of docetaxel in gastric tumors, thus causing an increase in anticancer activity [
189].
Ongoing research underscores the significance of the TME in driving tumor proliferation, invasion, metastasis, and resistance to therapeutic interventions. As mentioned, the TME provides protection for cancer cells, enabling them to evade conventional treatments like surgery, radiotherapy, and chemotherapy. Furthermore, the constituents of the TME play a pivotal role in fostering therapy resistance in solid tumors. Consequently, directing interventions toward the TME presents a promising avenue for advancing the field of cancer nanomedicine. The combination of antitumor drugs with drugs that interfere with resistance mechanisms has largely been made possible by advancements in nanotechnology [
190]. Hence, directing efforts toward the TME presents an innovative approach to advancing the field of cancer nanomedicine [
37,
191]. Nanoparticles developed in response to TME cues, such as a low pH, redox conditions, and hypoxia, enhance the pharmacokinetics and therapeutic effectiveness of nanomedicine, but also have glycolytic inhibitors [
37,
192,
193,
194]. Although not directly associated with this, and as has been shown for DCA in a lung cancer cell model, the use of nanoparticles improves the delivery of the compound, which can be important in cases of resistance.
Using NPs to direct therapy to energy metabolism and the TME could be a promising approach to sensitizing cells to conventional chemotherapy. Although the use of nanotechnology is still a recent field in cancer therapy, there is already enough evidence of its potential for successful treatment, allowing for a more accurate and specific delivery of antitumor drugs into cancer cells and avoiding many adverse side effects. Many barriers still need to be overcome regarding the success of NPs in clinical trials. Some of these barriers include the size and timing of certain NP therapies. The majority of experimental tests of NPs are cell-based and use animal models, which may not lead to convincing results in human testing. Furthermore, as the presence of metastases is a significant property of cancer, more studies should be carried out with models of cancer metastasis [
201].
5. Conclusions
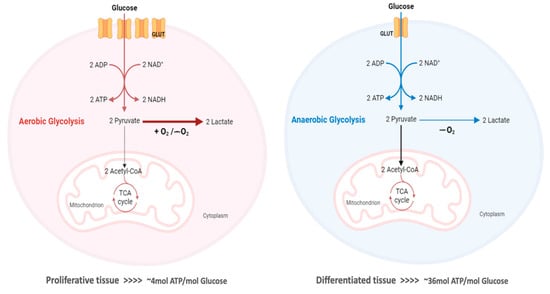
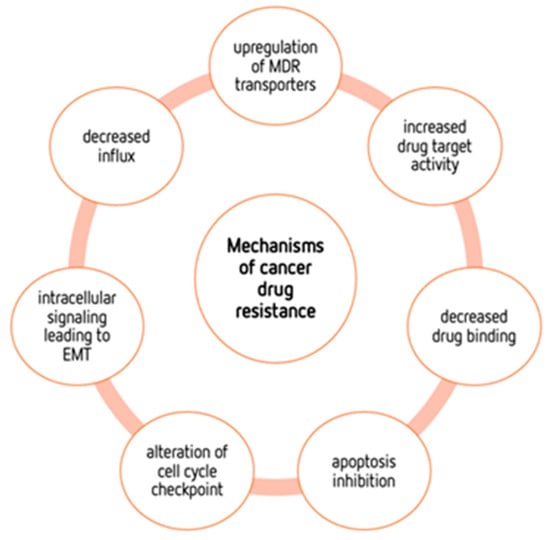
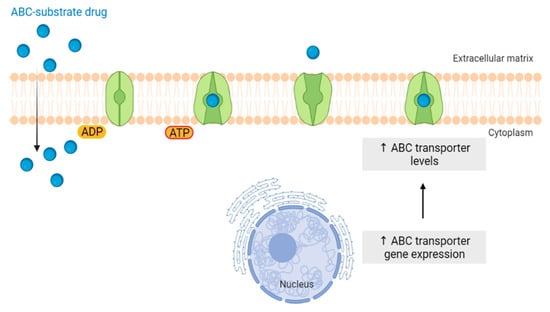
Although conventional chemotherapy is particularly toxic to tumor cells, it is often non-specific, and is responsible for the significant side effects associated with cancer treatment. However, there are differences between cancer cells and healthy cells that can be explored to increase treatment specificity against cancer. One of these differences consists of the “Warburg effect”, currently considered an emergent cancer hallmark, whereby the upregulation of the glycolytic rate in tumor cells is a key player in acid-resistant phenotypes through their adaptation to hypoxia and acidosis, as well as in tumor aggressiveness [2,9,159]. High glycolytic rates are widely reported to promote the chemoresistance of tumor cells to conventional therapy [2]. In fact, increased acidification of the extracellular space leads to lower drug stability and, consequently, lower drug efficacy. In parallel, the increased production of glycolytic intermediates promotes cell proliferation, since these are biosynthetic precursors, whereas ATP production sustains the activity of proteins involved in both drug efflux and cell division. Together, these effects underly multidrug resistance. Nevertheless, many cancer cells adapt to changes in TME, exhibiting metabolic plasticity and switching their metabolism from glycolysis to OXPHOS, and vice-versa. For example, OXPHOS could be the predominant metabolic pathway used by cancer stem cells, and is often involved in cancer resistance, metastasis, and tumor relapse [202]. Exploring specific characteristics of cancer cells, such as this change in metabolism, could be a promising strategy for the use of more effective and more specific drugs that primarily target cancer cells. In fact, metabolic changes in cancer cells can reveal specific vulnerabilities that could be targeted with precision therapies. However, the metabolic plasticity and interchange of glycolytic and oxidative cells, although occurring many times in the same cancer and being responsible for tumor heterogeneity, is not taken into account in cancer therapies. Thus, more integrated research is needed, investigating the main metabolic pathways used in different conditions and stages of each cancer type, and the influence of the TME characteristics (e.g., oxygen, pH, nutrients availability, immune components) on such metabolic adaptation and heterogeneity. An understanding of these metabolic switches, the identification of metabolic targets, and the use of combined therapies in a more targeted way through the use of nanoparticles could have a huge impact not only on the development of new drugs, but also on the ability to overcome drug resistance, one of the major problems that occurs during cancer treatment.