Chronic neuropathic pain (NP) is an increasingly prevalent disease and leading cause of disability which is challenging to treat. Several distinct classes of drugs are currently used for the treatment of chronic NP, but each drug targets only narrow components of the underlying pathophysiological mechanisms, bears limited efficacy, and comes with dose-limiting side effects. Multimodal therapies have been increasingly proposed as potential therapeutic approaches to target the multiple mechanisms underlying nociceptive transmission and modulation. However, while preclinical studies with combination therapies showed promise to improve efficacy over monotherapy, clinical trial data on their efficacy in specific populations are lacking and increased risk for adverse effects should be carefully considered. Drug-drug co-crystallization has emerged as an innovative pharmacological approach which can combine two or more different active pharmaceutical ingredients in a single crystal, optimizing pharmacokinetic and physicochemical characteristics of the native molecules, thus potentially capitalizing on the synergistic efficacy between classes of drugs while simplifying adherence and minimizing the risk of side effects by reducing the doses.
1. Introduction
Pain is an unpleasant sensation, with a salient emotional component, that can be an adaptive and critical alert system, facilitating learned avoidance of potential tissue damage or other danger [
1]. However, persistent, chronic pain becomes pathological and imposes substantial clinical, psychological, social, and financial burdens on society. Chronic pain is a critical global health concern, with chronic pain syndromes identified as one of the leading contributors to the global disease burden worldwide [
2]. Neuropathic pain (NP), which arises from a disease or lesion of the somatosensory nervous system [
1,
3], represents a substantial proportion of chronic pain, with nearly one quarter of those with chronic pain and up to 10% of the global population experiencing chronic NP [
4].
NP significantly impairs the quality of life and daily activities of patients, resulting in total health-care costs increasing to three times higher than in matched controls [
5]. The underlying etiology of NP is multimodal and heterogenous, making its diagnosis and therapeutic management extremely challenging. Further complicating the picture, currently available analgesics often only convey partial pain relief for NP patients and are associated with dose-limiting side effects [
6,
7,
8,
9].
Effective approaches for the pharmacological management of NP will be those that leverage synergistic efficacy associated with multimodal therapies acting on converging mechanisms underlying NP in parallel. However, the wide-spread use of traditional combination therapy regimens is hindered by dose-limiting side effects and limited efficacy and safety data supporting the risk/benefit profiles for combinations proposed thus far.
2. Pathophysiological Mechanisms of Chronic NP
Canonically, nociception is the activation of multimodal receptors, nociceptors, which transduce noxious stimuli into electrical signals and conduct from peripheral sensory nerves to the central nervous system (CNS) to drive the sensory experience of pain [
10]. Types of pain are classified according to the pathophysiological mechanisms of nociceptor activation and duration. When pain reflects the mechanism and the severity of a sporadic and limited etiologic event, it can be defined as acute; but if it occurs regularly over a period of several months (i.e., more than 3 months), the pain process becomes chronic and pathological [
3,
11]. Nociceptive pain occurs when tissue damage (e.g., following internal or external injuries originating from accidents or medical procedures) activates nociceptors [
10]. A newly defined category of pain, known as nociplastic pain, is characterized by the absence of obvious signs of sickness, injury, or somatosensory system lesion. In this case, pain arises from altered nociception and central sensitization, and the nervous system plays a central role [
12].
The definition of NP has been elusive for the last 20 years. Its diagnosis and management remain difficult and to some extent arbitrary today. This is due in part to a lack of biomarkers and/or clinical tests, and the extremely dynamic nature of the nociceptive system. In 2019, the International Association for the Study of Pain (IASP) published a classification system to facilitate epidemiological investigations and health policy decisions surrounding the research and availability of multimodal pain management [
3,
11].
NP is usually chronic, defined as persistent or recurrent increased pain sensitivity or spontaneous pain stemming from a lesion or disease to the peripheral or central nervous system [
1,
3,
11]. Somatosensory abnormalities in an area innervated by the damaged peripheral or central nerves are the primary complaint of patients with NP [
11]. Symptoms affect both spontaneous and evoked pain perception and can include hyperesthesia, hypoesthesia, allodynia, and paresthesia. In addition to the presence of these symptoms and a pain syndrome, peripheral and/or central changes in one or more of the somatosensory systems, including non-nociceptive tactile and proprioceptive systems, thermoregulatory systems and visceral afferents, confirmed by clinical tests (e.g., electromyography, nerve or skin biopsies, CNS imaging, biochemistry and molecular biology), are required to receive a diagnosis of NP [
1,
13]. The etiology is multifactorial/multifaceted, as diseases or lesions throughout the entire pain perception path, from the peripheral nociceptor to the cortex, can drive NP syndromes. Peripheral NP can be caused by genetic mutations in key nociceptive receptors or ion channels or by a variety of axonal or demyelinating injuries [
14]. Peripheral NP occurs due to acute events (e.g., trauma, amputation, and surgery), diseases (e.g., diabetes, and herpes zoster) infection/post-infection syndromes (e.g., Guillain-Barre syndrome), peripheral entrapment, spinal column degeneration, tumor infiltration, or adverse events associated with pharmacological or radiation treatments [
14,
15,
16]. In contrast, central NP can be caused by lesions in the spinal cord, the spinothalamic tract, or the brain, with specific involvement of the brainstem, thalamus, and subcortical structures [
1]. Common etiologies of central NP include post-stroke pain, multiple sclerosis, Parkinson’s disease, Alzheimer’s disease, amyotrophic lateral sclerosis, and syringomyelia. NP can also be classified as mixed NP when it is derived from a combination of peripheral and central somatosensory impairments, for example, as in lumbar or cervical radiculopathies [
17,
18,
19].
3. Pharmacological Treatment of Chronic NP: Drug Classes/Monotherapy
Currently available pharmacological treatments used for the management of chronic NP are only able to mitigate the severity of symptoms rather than address the causative factors driving the disease [
20]. According to the most recent evidence-based recommendations from the Neuropathic Pain Special Interest Group (NeuPSIG, Washington, DC, USA), first-line recommended treatments for NP include certain antidepressants and antiepileptic drugs, with tramadol and topical treatments recommended as first-line treatment if the pain is localized or as second-line treatment [
7,
20,
21]. Third-line treatments include strong opioids or botulinum toxin type A-haemagglutinin complex (BoNTA).
First-line antidepressants recommended for the treatment of peripheral or central NP include tricyclic antidepressants (TCA) (amitriptyline and nortriptyline) and serotonin-noradrenaline reuptake inhibitors (SNRI) (duloxetine and venlafaxine) [
7,
20,
21]. Despite a strong GRADE (Grading of Recommendations Assessment, Development and Evaluation) recommendation (SNRIs, high-quality evidence; TCAs, moderate-quality evidence), analyses indicated only moderate effect sizes or low to moderate tolerability [
7]. TCAs and SNRIs were initially developed for the treatment of mood disorders. The mechanisms through which they exert analgesia are still under investigation, but are thought to stem from descending noradrenergic signaling and/or N-methyl-D-aspartate receptor and ion channel blockade [
22].
Evidence supporting the use of the first-line recommended antiepileptic drugs, gabapentinoids, or GABA-mimetic antiepileptic drugs [
23], is the strongest of those assessed by the NeuPSIG and applicable to both central and peripheral NP (strong GRADE recommendation, high-quality evidence, moderate effect size, moderate to high tolerability) [
7,
20]. Gabapentinoids, such as gabapentin and pregabalin, are first- and second-generation α2δ inhibitor ligands, respectively, and both are approved for use as adjunctive therapy in pain control. Their analgesic efficacy is thought to stem from the inhibition of injury-induced spinal neuronal excitability via the α2δ subunit of pre-synaptic calcium channels in the spinal cord. They can also act at the supraspinal level by modulating the dopamine-dependent negative affective states that have been reported in pain [
24] or modulating the release of substance P-induced neurotransmitter release [
25].
Tramadol (an opioid with additional monoaminergic activity [
30,
31]) and topical treatments (if the pain is localized), such as capsaicin high-concentration patches and lidocaine, may be used as a first-line or second-line treatments but are associated with weaker effect sizes [
7,
21]. Specifically, tramadol is recommended for central or peripheral NP and is associated with a weak GRADE recommendation and moderate-quality evidence, while capsaicin patches and lidocaine are recommended for peripheral NP and associated with weak GRADE recommendations based on either high-quality or low-quality evidence, respectively [
7]. Strong opioids are recommended as third-line treatments for both peripheral and central NP, but this is a weak GRADE recommendation based on only moderate-quality evidence and recent increases in prescription opioid-related overdose deaths emphasize the gravity of the abuse liability associated with opioid use [
7,
32,
33]. The development of tolerance to opioids, counteracting the effects of repeated administrations, also limits their long-term use in patients with NP. Lastly, Botulinum toxin type A-haemagglutinin complex (BoNTA) may help some NP patients, but is only recommended for peripheral NP (weak GRADE recommendation, low-quality evidence) [
7].
Nonsteroidal anti-inflammatory drugs (NSAIDS), or nonselective cyclo-oxygenase-1/2 (COX1/2) inhibitors, as well as selective COX2 inhibitors, are also commonly used to manage inflammation and pain in NP, particularly in sub-acute and acute-on-chronic phases [
34,
35]. NSAIDs exert efficacy both by controlling peripheral inflammation and through either the disinhibition of endogenous opioid signaling or activation of endocannabinoid signaling at the level of the spinal cord, periaqueductal gray (PAG) and rostral ventromedial region of the medulla [
36,
37,
38,
39,
40]. However, tolerability of NSAIDs and selective COX2 inhibitors is limited by their risk for renal toxicity, gastrointestinal toxicity, cardiotoxicity, and nephrotoxicity [
41,
42,
43,
44], discouraging their use in patients with pre-existing pathologies [
45,
46,
47,
48,
49,
50].
While these recommendations highlight pharmacological agents with potential efficacy in some NP patients, it is well known that the benefits of single-drug treatments are limited due to incomplete efficacy and dose-restricting adverse effects [
6,
7,
8,
9]. High risks of dose-related adverse reactions prevent the use of doses sufficient to produce complete pain relief [
7]. Complicating the picture, risks for adverse effects may also differ depending on the specific etiologies of NP and need to be considered on an individual patient level [
7]. The lack of clear efficacy of monotherapies in NP can be explained by the converging mechanisms and multiple potential pathologies known to contribute to chronic NP [
52].
4. Pharmacological Treatment of Chronic NP: Combination Therapy
4.1. General Considerations
A multimodal approach to chronic NP management allows the modulation of multiple transmission pathways and enables individual pharmacological agents to act with potentially additive or synergistic effects (i.e., improvements in efficacy greater than the theoretical summation of the two analgesic effects). Drugs with distinct pharmacological mechanisms of action acting at different sites of nociceptive signaling will optimize efficacy [
54,
55]. Ideally, two distinct classes of drugs used in combination will have non-overlapping side effect profiles and minimal potential for either adverse interactions with other commonly used drugs or for the exacerbation of existing patient comorbidities [
56,
57]. When synergistic improvements in efficacy also render smaller doses of a given agent sufficient to achieve pain relief, combination therapy could also lead to improved tolerability [
58,
59,
60,
61].
Collectively, approximately half of patients with NP are reported to receive at least two analgesic drugs for pain management [
62,
63,
64]. Different combinations of NSAIDs, gabapentinoids, opioids, and antidepressants have been investigated so far and have shown some efficacy for the management of NP across syndromes [
65,
66,
67], and specifically for postoperative pain [
68,
69,
70,
71], rheumatological conditions (e.g., osteoarthritis or fibromyalgia) [
72,
73,
74,
75,
76], diabetic neuropathy [
77,
78], back pain [
79], post-herpetic neuralgia [
80], and cancer-related pain or chemotherapy-induced peripheral neuropathy [
81,
82]. However, preclinical and clinical data describing the safety and efficacy of combination therapies are inconsistent [
83,
84]. Fine-tuning the balance between positive and adverse drug-related effects in all pain conditions, as well as consideration of individual patient characteristics (i.e., response to previous analgesics, the duration and severity of symptoms, and the presence of comorbidities) and the pathogenic mechanisms driving the NP are crucial for the success of combination therapies. Preclinical studies in which animal responses to noxious stimuli (e.g., heat, electrical current, or mechanical stress) or inflammatory pain (e.g., peripheral injections of carrageenan, formalin, complete Freund’s adjuvant, or monoiodoacetate) engage distinct underlying pathophysiological mechanisms are useful to gauge the preclinical efficacy of candidate combination therapies in this context [
85,
86,
87,
88,
89].
4.2. Preclinical and Clinical Data on Combination Therapy
4.2.1. NSAIDs and Opioids (Including Tapentadol/Tramadol)
NSAIDs are widely used in the treatment of various inflammatory pain states, including some NP syndromes. Because of their well-established, dose-limiting gastric and cardiovascular tolerability issues, they are often combined with analgesics of several classes that act through different mechanisms. The combination of dexketoprofen and tramadol has been identified as a valuable combination with synergistic improvements in analgesic efficacy. Tramadol acts as a weak μ-opioid receptor agonist and as a serotonin/noradrenaline reuptake inhibitor [
30,
31]. This combination leverages analgesic efficacy of relatively weak opioid activity in the spinal cord, enhanced descending noradrenergic contributions to pain inhibition, tramadol-driven modulation of microglia, and the anti-inflammatory activity characteristic of NSAIDs [
72,
90]. The combination of dexketoprofen and tramadol was associated with synergistic efficacy in preclinical models of nociceptive/postoperative (plantar incision) pain (i.e., tail flick, acetic acid writhing test, von Frey) [
90,
94] and inflammatory/chronic osteoarticular pain [
95]. However, analyses of gastrointestinal transit following this combination revealed an increased risk for constipation, with dexketoprofen reversing the effects of tramadol in this context [
94].
4.2.2. NSAIDs and Gabapentinoids/Antiepileptics
Several studies have evaluated analgesia following combinations of NSAIDs with antiepileptics, including gabapentin and pregabalin. With this combination, in addition to peripheral NSAID anti-inflammatory activity counteracting central sensitization and pain maintenance, spinal neurotransmitter spill over is mitigated by the gabapentinoid-induced inhibition of the α2δ subunit of presynaptic calcium channels. A known mechanism through which gabapentinoids and NSAIDs may interact is through the inhibition of the membrane translocation of protein kinase C epsilon type (PKC𝜀) in sensory neurons. This is an inflammatory-dependent process underlying pain maintenance [
130], and, notably, paracetamol has been shown to potentiate the gabapentin-mediated blockade of PKC𝜀 membrane translocation induced by the pronociceptive peptides, bradykinin and prokineticin 2 in vitro [
131].
Synergistic efficacy was observed in preclinical models of localized inflammatory pain (i.e., formalin- or carrageenan-induced) with several gabapentinoid/NSAID combinations including gabapentin–naproxen [
97], pregabalin–naproxen [
97], gabapentin–ibuprofen [
98], and oxcarbazepine–ibuprofen [
99]. The synergistic boost in efficacy from coadministration of ibuprofen with oxcarbazepine resulted in a reduction in the effective dose required to produce 50% analgesic efficacy (ED
50) from a theoretical additive ED
50 of 67.63 ± 5.44 mg/kg to an experimentally observed ED
50 of 35.71 ± 2.90 mg/kg. A reduction in the dose required to produce sufficient analgesia with these combinations could substantially reduce the risk of adverse effects for patients with NP [
99], but clinical studies are needed to investigate whether this synergistic efficacy translates clinically.
Combination therapy with gabapentin–diclofenac exhibited synergistic efficacy in both postoperative and inflammatory pain models. Intrathecal coadministration of gabapentin and diclofenac at low doses of each agent insufficient to produce anti-hyperalgesia when administered alone (gabapentin, 4 µg; diclofenac, 2 µg) significantly reduced mechanical hyperalgesia when administered in combination in a preclinical model of postoperative pain (hindpaw incision) [
100]. Similarly, synergistic efficacy was observed with this combination in inflammatory pain (i.e., formalin-induced), with an improvement in the ED
30 value for diclofenac–gabapentin (administered directly into the hind paw) from the theoretical additive value of 597.5 ± 87.5 µg to the experimentally observed value of 170.9 ± 26.07 µg [
101].
Synergistic efficacy greater than the theoretical sum of the effects produced by each drug alone was also observed in rat models of chronic constriction injury (CCI) and SNI with combinations of NSAIDs and gabapentinoids. In a rat model of CCI, synergistic anti-allodynic and anti-hyperalgesic effects were observed following combination therapy with meloxicam (1.0 mg/kg) and gabapentin (10 mg/kg) [
102].
4.2.3. Gabapentinoids and Opioids (Including Tapentadol/Tramadol)
In a preclinical study examining the effects of various combinations of pregabalin with duloxetine, venlafaxine, tramadol, and celecoxib on mechanical allodynia in rats with an L5 spinal nerve ligation, pregabalin with tramadol was the only combination to show synergistic anti-allodynic effects [
103]. In the same study, pregabalin and duloxetine exhibited additive, but not synergistic efficacy, while pregabalin and venlafaxine appeared to be antagonistic. Of note, although combination treatment in the management of chemotherapy-induced peripheral neuropathy (CIPN) is still controversial, combination therapy with pregabalin and tramadol was found to have positive outcomes in taxane-induced peripheral neuropathy [
82].
There is preclinical and clinical evidence supporting a synergistic effect of the combination of gabapentin and stronger opioids as well [
104,
105]. Synergistic anti-allodynic and anti-hyperalgesic effects of combination therapy with gabapentin and morphine greater than either agent administered as monotherapy or the theoretical additive effect of the combination were observed following CCI with the von Frey and acetone tests in an NP model in rats [
105]. Gabapentinoid–opioid combinations have been evaluated also in several clinical studies in various NP patient populations; however, efficacy results have been inconsistent and safety and tolerability concerns remain about the risks for side effects with low-dose pregabalin and opioids [
65,
66].
Despite the observed efficacy of several single drugs in diabetic peripheral neuropathic pain (DPNP), only approximately half of patients respond to treatment, with many reporting residual symptoms [
78,
132]. One randomized, double-blind, placebo-controlled trial demonstrated a significant improvement in pain relief with the addition of oxycodone to gabapentin treatment, relative to gabapentin treatment alone, in patients with DPNP [
78].
Importantly, while the addition of gabapentin to an existing opioid regimen for the treatment of severe pain may lower the opioid dose required for pain relief, it brings with it additional risk for adverse effects associated with gabapentin. A 2022 meta-analysis of the risk of adverse events associated with opioid therapy vs. combination opioid and pregabalin therapy revealed that combination therapy may increase the risk for CNS depression and mortality, despite a tolerable gastrointestinal profile [
66].
The combination of morphine with pregabalin markedly reduced pain scores relative to either agent as monotherapy in patients with several forms of NP, including back surgery syndrome, post-herpetic neuralgia, radiculopathy and stenosis of the spinal medullary canal [
106]. This effect was achieved with lower mean dosages of morphine and pregabalin relative to doses required for monotherapy with each agent. Consistent with this, in a multicenter trial including patients with NP syndromes (i.e., back surgery syndrome, stenosis of the medullary spinal canal, post-herpetic neuralgia, and painful diabetic neuropathy), the combination of pregabalin and oxycodone was superior to pregabalin alone and caused fewer adverse effects than either oxycodone alone or pregabalin alone, allowing a dose reduction of both medications compared to the respective effective doses required for monotherapy [
67].
4.2.4. Gabapentinoids and Antidepressants
Combination therapy with gabapentinoids and antidepressants has also been examined in the context of DPNP, with inconclusive results. Patients with diabetic neuropathy treated with gabapentin in combination with venlafaxine reported pain relief and improvements in quality of life compared to those in a placebo group and to those who received gabapentin and placebo [
77].
4.2.5. Additional Combination Therapies
In preclinical studies, combination therapy with metformin, a widely used first-line antihyperglycemic agent for type 2 diabetes, and ibuprofen, aspirin, tramadol or pregabalin led to a significantly better reduction of carrageenan-induced hyperalgesia as compared to monotherapy of each compound [
111]. Similar synergistic analgesia was observed with diclofenac coadministered with the H1 antihistaminic pyrilamine in a rat model of carrageenan-induced paw edema [
112]. In this study, in addition to synergistic improvements in anti-inflammatory and anti-nociceptive efficacy, diclofenac and pyrilamine combination therapy reduced gastric liability.
Gabapentin-metamizol [
117], and oxcarbazepine–paracetamol combinations also exhibited anti-nociceptive improvements in models of inflammatory pain (i.e., formalin- or carrageenan-induced) [
99,
118]. In a partial spinal nerve injury model, combination therapy with subeffective doses of gabapentin and pregabalin potentiated the therapeutic efficacy of spinal cord stimulation, a tool used for neuropathic pain relief, as evaluated via the von Frey test [
119]. Extracellular recordings confirmed that this low-dose combination enhanced the suppression of dorsal horn neuron hyperexcitability.
Cannabinoids have been proposed as useful analgesics with weaker central psychological effects than opioids and have been evaluated in combination therapy regimens [
133]. Indeed, one study demonstrated that intra-plantar coadministration of anandamide and ibuprofen or anandamide and rofecoxib ameliorated mechanical allodynia and thermal hyperalgesia in a model of partial sciatic nerve ligation, and suggested local use of cannabinoids as a potential way to take advantage of their anti-nociceptive nature and limit unwanted central effects [
120].
Preclinical evidence also supports further evaluation of the potential for combination therapy with cannabinoids and opioids in the treatment of NP [
133]. Combination therapy with morphine and WIN55,212-2 in rats exposed to chronic constriction injury [
124] and combination therapy with tramadol and PhAR-DBH-Me, a synthetic cannabinergic compound, in rats exposed to spinal nerve ligation [
125] both led to synergistic analgesic efficacy.
The coadministration of ibuprofen and paracetamol in fixed-dose combinations (FDC) has been suggested for pain management and has demonstrated synergistic efficacy in several contexts. It has been recommended as a preferred option relative to either agent as monotherapy for the treatment of headaches, odontalgia, earache, musculoskeletal pain, and postoperative pain in pediatric patients [
134]. This combination has also been evaluated for pain management in adult patients. While a meta-analysis of nine clinical trials revealed a significant improvement in outcomes of patients treated with FDC ibuprofen and paracetamol relative to placebo, its value relative to either administered as monotherapy was not evaluated and safety analyses were inconclusive [
135].
4.3. Unmet Medical Needs
Collectively, preclinical and clinical studies have provided a proof of concept and shown promise for some multimodal combination therapy regimens to convey additive or synergistic improvements in efficacy for NP. This represents an important achievement in the field of NP management warranting further investigation. However, there are several obstacles remaining before any specific combination therapy can be widely used for NP. The risk of side effects is still a substantial limiting factor, with a lack of clear safety and efficacy data and substantial risk for adverse events in specific NP populations.
5. Pharmacological Treatment of Chronic NP: Co-Crystallization
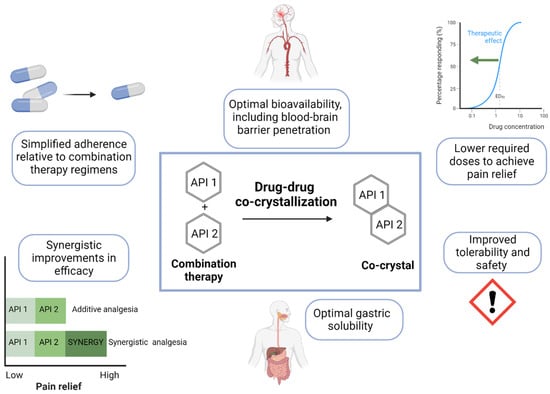
Co-crystallization has emerged in pharmaceutical science not only as solution for poorly soluble drugs, but also as an innovative pharmacological approach to drug combination which allows for the optimization of physicochemical and pharmacokinetic properties. In drug-drug co-crystallization, the aggregation of two or more active pharmaceutical ingredients (APIs) via noncovalent interactions yields a new single crystal with unique physicochemical properties but the same intrinsic chemical identity and pharmacological activity of the native molecules [
138,
139]. Thus, potentially, drug–drug co-crystals can leverage the synergistic efficacy of distinct classes of drugs while simultaneously optimizing bioavailability and minimizing the required doses and associated side effects, providing significant advantages over combination therapy regimens (
Figure 1).
Figure 1. Potential advantages of co-crystals in the treatment of chronic NP. Drug-drug co-crystals can convey distinct advantages over combination therapy regimens including simplified adherence, optimal bioavailability and blood–brain barrier penetration, lower doses, improved tolerability, improved solubility, and synergistic improvements in analgesic efficacy. API, active pharmaceutical ingredient.
Novel co-crystals formed from two analgesics have been synthesized. Two recent reports of novel co-crystals have demonstrated improved physicochemical properties that would be expected to convey benefits in vivo once evaluated. A co-crystal system containing febuxostat and piroxicam, both approved for the treatment of gout, exhibited improved solubility of both febuxostat and piroxicam and an improved dissolution rate of piroxicam [
140]. In another example, co-crystallization of meloxicam with aspirin led to significantly improved meloxicam kinetic solubility, with improved oral bioavailability and higher maximum plasma concentration, which could substantially improve the time required for the drug to reach therapeutic concentrations [
141]. Finally, drug–drug co-crystals of duloxetine and naproxen [
142], diclofenac acid and ethyl diclofenac [
143] or anti-inflammatory agents like ibuprofen, naproxen, ketoprofen, and flurbiprofen with levetiracetam [
144] have also been reported.
Two novel co-crystals have been evaluated in vivo for pain management. Almansa and colleagues were the first to report the development of co-crystals containing rac-tramadol hydrochloride and celecoxib and demonstrate synergistic efficacy in vivo [
145,
146]. Tramadol and celecoxib represent distinct drug classes with complementary mechanisms targeting multiple central and peripheral NP pathways, with celecoxib being suggested to have improved cardiovascular safety relative to other NSAIDs [
147,
148]. The co-crystal, called Seglentis
® has been evaluated for the treatment of acute postoperative pain. In this context, it exhibited an improved pharmacokinetic profile, with a faster intrinsic dissolution rate of celecoxib relative to celecoxib alone and a slower intrinsic dissolution rate of tramadol relative to tramadol alone [
145,
146]. The lower peak concentration of tramadol and faster analgesic onset of celecoxib in the co-crystal is an advantage over combination therapy of the two agents as it could convey a substantially improved risk/benefit ratio. Consistently, the co-crystal was more effective than the single drugs, and more effective than the theoretically calculated additive benefit of the two drugs, in reducing acute postoperative pain without potentiating the risk for adverse effects [
145,
146,
149].
Phase I clinical studies confirmed that the co-crystal was associated with the expected improved pharmacokinetic profile relative to either of the individual formulations or their combination [
150]. Subsequently, Phase II and III studies confirmed the significant improvement in the risk/benefit ratio observed with the co-crystal relative to either agent alone [
151,
152,
153]. Seglentis
® is now approved for the treatment of acute pain in adults that is severe enough to require an opioid analgesic for which other treatments are inadequate [
154].
6. Conclusions
The heterogenous and multimodal nature of chronic NP makes it a very challenging condition to treat. The underlying etiology of a given peripheral or central NP condition is important to consider in selecting optimal treatment regimens or assessing new data regarding novel therapeutic approaches like those described here. Currently available analgesics are associated with significant safety concerns, including renal toxicity, gastrointestinal toxicity, cardiotoxicity, nephrotoxicity, and abuse liability, underscoring the need to fine-tune the balance of the risk/benefit ratio and closely monitor the tolerability of doses and durations of current treatment prescribed. Multimodal therapeutic approaches represent an important step forward in improving patient outcomes. Drug-drug co-crystallization has the potential to offer several key advantages over combination therapy regimens in this context, improving physicochemical and pharmacokinetic properties of the existing molecules and conveying consequent higher efficacy and/or reduced dose requirements superior to those achieved with combination therapy of the single agents. Critically, the selection of APIs with pharmacodynamic profiles suitable for synergistic efficacy and physicochemical properties which align with stoichiometric constraints is challenging [159]. There are limited examples of drug-drug co-crystals in the pain area that have been published thus far [145].
This entry is adapted from the peer-reviewed paper 10.3390/biom13121802