Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Endometriosis is an inflammatory and estrogen-dependent condition, affecting approximately 6–10% of women in their reproductive years. The pathogenesis and histological findings are characterized by endometrial glandular and stromal tissue growing beyond the uterus. It is classified into three types: superficial, ovarian, and deep endometriosis. In addition to gynecological manifestations, many endometriosis patients experience gastrointestinal symptoms, indicating a potential association between gut health and the disease.
- endometriosis
- gut microbiota
- estrogens
- dysbiosis
- chronic inflammation
1. Introduction
Endometriosis is an inflammatory and estrogen-dependent condition, affecting approximately 6–10% of women in their reproductive years [1][2]. The pathogenesis and histological findings are characterized by endometrial glandular and stromal tissue growing beyond the uterus. It is classified into three types: superficial, ovarian, and deep endometriosis [3][4]. Although it is not considered a malignant disease, it can cause debilitating symptoms such as dysmenorrhea, dyspareunia, dysuria, and dyschezia (the 4-D’s) (Figure 1A) [5], and it may affect the fertility, psychological well-being, and social functioning of the patients. Moreover, it is one of the leading causes of access to the emergency department [6].
The growth of endometrial implants and the associated inflammation lead to increased production of pro-inflammatory cytokines [7][8][9][10]. These processes eventually lead to chronic inflammation, forming pelvic adhesions and endometriomas, which disrupt anatomical structures and impair reproductive function [9][11]. In addition to gynecological symptoms [12][13], up to 90% of patients with endometriosis experience gastrointestinal symptoms [14] (Figure 1A). Common gastrointestinal symptoms include bloating, nausea, constipation, diarrhea, and vomiting [14][15][16]. Recent advancements in genomics research and high-throughput sequencing technology have provided valuable insights into the relationship between human microbiota and female reproductive health [17][18]. It has been documented that the gut microbiota plays a significant role in various inflammatory conditions [19], and numerous studies have confirmed this association.
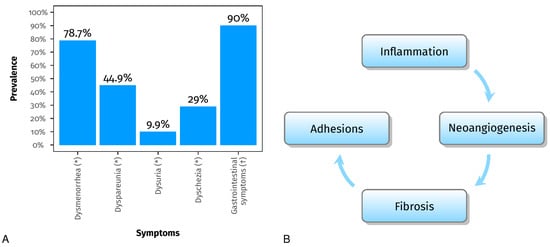
Figure 1. Panel (A) shows the prevalence of dysmenorrhea, dyspareunia, dysuria, and dyschezia (the 4-D’s) (*) [20] and the prevalence of gastrointestinal symptoms (†) [14]. Panel (B) shows the processes involved in endometriosis, which can disrupt anatomical structures and impair reproductive function [7][8][9][10][11].
2. Gut Microbiota and Estrogens
Considering the immunological and hormonal alterations observed in individuals with endometriosis and the influence of the gut microbiota on immune and estrogen responses, there is a hypothesis that the gut microbiota plays a role in the development of endometriosis [19][21][22][23].
The gut microbiota can secrete enzymes such as β-glucuronidase and β-glucosidase, which can deconjugate estrogens and increase the reabsorption of free estrogen, leading to higher estrogen levels in the bloodstream [24][25][26][27][28][29].
The collection of genes encoding estrogen-metabolizing enzymes in the gut microbiota is commonly known as the “estrobolome” [30].
Analysis of the microbial genome has revealed that several bacterial genera in the gut microbiota, including Bacteroides, Bifidobacterium, Escherichia coli, and Lactobacillus, can produce β-glucuronidase [31].
Interestingly, research has shown a significant increase in Escherichia coli levels in the feces of endometriosis patients [32]. These findings suggest that the gut microbiota may contribute to elevated estrogen levels, creating an environment that promotes the progression of endometriosis [19][33].
Finally, in a study published in 2023, it seems that gut microbial β-glucuronidase (gmGUS) can become a potential biomarker for the early diagnosis of estrogen-induced diseases [34].
3. Dysbiosis and Chronic Inflammation
In 2002, an animal model study of female Rhesus Macacus with endometriosis showed a different microbiota composition than the healthy sample [35]. It appears that endometriosis was linked to reduced concentrations of Lactobacilli and elevated concentrations of Gram-negative bacteria. Furthermore, a higher incidence of intestinal inflammation was observed in monkeys with endometriosis compared to the healthy control group (the prevalence of intestinal inflammation in monkeys with or without endometriosis was determined through a retrospective analysis of necropsy reports).
The objective of the study by Svensson et al. [16] was to compare the gut microbiota of individuals with endometriosis and healthy controls. The researchers investigated whether there were variations in microbiota abundance within the endometriosis group based on disease localization, symptoms, or treatment. The study found a significant difference in overall gut microbia between healthy controls and individuals with endometriosis, with the gut microbiota of healthy controls showing higher diversity compared to patients with endometriosis. This finding suggests that there may be an association between reduced gut microbial diversity and the presence of endometriosis.
Imbalances in the composition of gut microbiota, known as dysbiosis, can disturb regular immune function, resulting in increased levels of proinflammatory cytokines, compromised immunosurveillance, and changes in immune cell profiles. These immune dysregulations are implicated in the chronic inflammatory state observed in endometriosis. The chronic inflammation associated with endometriosis creates an environment that promotes increased adhesions and angiogenesis, critical disease features. Adhesions can cause organs and tissues to stick together, leading to pain, functional impairment, and the development of new blood vessels that can provide oxygen and nutrients to the endometrial lesions, allowing them to grow and spread [11][36]. A study by Huang et al. [37], which compared the microbiome of peritoneal fluid, stool, and cervical mucus in endometriosis patients and healthy controls, observed an increased abundance of Gram-negative bacteria in the endometriosis group, including Pseudomonas and Prevotella, with the potential to release lipopolysaccharide (LPS). In the context of endometriosis, LPS can stimulate the activation of macrophages, leading to the production of inflammatory cytokines and chemokines. These inflammatory mediators can further promote the proliferation of endometriotic stromal cells, contributing to the growth and progression of endometriotic lesions [38].
4. Insights from Human and Animal Studies
The observed alterations in the microbiota of the gut, peritoneal fluid, and female reproductive tract in individuals with endometriosis compared to healthy controls have been well-documented in various human and animal studies, and at the same time, many experimental animal models have supported a bidirectional relationship between endometriosis and microbiota changes [11][16][37][39][40][41][42][43][44][45][46]. However, it is still not fully understood whether these alterations are a consequence of endometriosis or if they play a causative role in the development of the disease [47].
The findings of the main studies investigating the association between gut microbiota and endometriosis in animals and humans are summarized in Table 1 and Table 2, respectively.
A study in mice with surgically induced endometriosis has shown that antibiotic treatment can reduce the size of endometriotic lesions, suggesting a potential role for the gut microbiota in disease progression. Additionally, fecal microbiota transfer from endometriotic mice to healthy mice resulted in the regrowth of lesions and associated inflammation [41]. Yuan et al. [45] conducted a prospective and randomized experiment using an animal model of endometriosis induced by intraperitoneal injection of endometrial tissues. Mice were divided into two groups: endometriosis and mock. The mice were sacrificed at four different time points to validate the model and collect fecal samples. 16S ribosomal-RNA gene sequencing was conducted to analyze the gut microbiota. The study found that the endometriosis and mock mice initially exhibited similar diversity and richness in their gut microbiota. However, after 42 days of modeling, distinct gut microbiota compositions were observed. Notably, mice with endometriosis showed an elevated Firmicutes/Bacteroidetes ratio, suggesting the induction of dysbiosis by endometriosis.
In their human studies, Hernandes et al. [42] aimed to identify and compare bacterial patterns in endometriotic lesions, eutopic endometrium, and vaginal fluid from endometriosis patients to those found in the vaginal fluid and eutopic endometrium of control patients. Amplicon sequencing results showed similar microbial profiles in vaginal fluid, eutopic endometrium, and endometriotic lesions, with the most abundant genera being Lactobacillus, Gardnerella, Streptococcus, and Prevotella. No significant differences were observed in the diversity analysis of microbiome profiles between control and endometriotic patients. However, deep endometriotic lesions appeared to have a distinct bacterial composition, with a lower predominance of Lactobacillus and a higher abundance of Alishewanella, Enterococcus, and Pseudomonas.
The study by Khan et al. (2021) [48] investigated the impact of antibiotic treatment, with or without GnRHa, on intrauterine infection and subsequent tissue changes in endometriosis. They collected endometrial/endometriotic samples from 53 women with endometriosis and 47 control women who received levofloxacin (LVFX) or GnRHa before surgery. The researchers used molecular and immunohistochemical analyses to assess the bacterial presence and various cellular markers. Results indicated that both LVFX and GnRHa + LVFX treatments significantly reduced specific bacterial genera in women with endometriosis. Specifically, in women with endometriosis, these treatments decreased Gardnerella, Prevotella, Acidibactor, Atopobium, Megasphaera, and Bradyrhizobium. Furthermore, the combination of GnRHa + LVFX demonstrated a greater reduction in chronic endometritis compared to GnRHa alone. These changes correlated with decreased macrophage infiltration (CD68), cell proliferation (Ki-67), and micro-vessel density (CD31) in endometria and endometriotic lesions, indicating an improved histological appearance of ovarian endometrioma. In conclusion, the study indicates that clinical administration of broad-spectrum antibiotics, with or without GnRHa, can be effective in reducing uterine infection and associated inflammation, cell proliferation, and angiogenesis in human endometriosis.
These results suggest that there is a dynamic interaction between the microbiota and endometriosis, where alterations in the microbiota can influence the development and progression of the disease, and conversely, the presence of endometriosis can affect the composition and function of the microbiota [49]. This dynamic relationship holds potential implications for non-invasive diagnostics and bacterial-based treatments in the future.
Table 1. Animal studies that explored the bidirectional relationship between gut microbiota and endometriosis.
| Study | N | Animal Species | Endometriosis Model | Sample | Technic | Aim and Conclusion |
|---|---|---|---|---|---|---|
| Bailey & Coe, 2002 [35] | 18 | Female Rhesus Monkeys (Macaca Mulatta) | Spontaneous occurring endometriosis, diagnosed with USG and MRI and confirmed by surgery and histology | Fecal samples | Coprocolture | Aim: demonstrate that monkeys have an altered profile of intestinal microflora, in particular altered Lactobacilli Conclusion: decreased Lactobacilli and increased gram-negative anaerobes and facultative anaerobes |
| Yuan et al., 2018 [45] | Cases: 16 Controls: 20 |
C57BL6 mice | Induced: intraperitoneal injection of endometrial tissue from sacrificed donor mice | Fecal pellets | NGS Illumina: V4 16S rRNA | Aim: determine the changes in gut microbiota in a murine endometriosis model by 16S ribosomal-RNA gene sequencing Conclusion: Firmicutes/Bacteroidetes ratio was elevated in mice with endometriosis, indicating that endometriosis may induce dysbiosis |
| Chadchan et al., 2019 [41] | Cases: 5 with endometriosis treated with vehicle and 4 with endometriosis and treated with broad spectrum antibiotics Controls: 5 |
C57BL/6 mice | Induced: autologous transplantation of endometrial tissue onto the peritoneal wall | Fecal pellets, peritoneal fluid, endometriotic lesions | NGS Illumina: V1-V9 16S rRNA | Aim: investigate if altering gut microbiota with antibiotic treatment has any impact on endometriosis progression Conclusion: oral antibiotic treatment is effective in reducing the progression of endometriosis and administering fecal material from mice with endometriosis via oral gavage restored both the growth of endometriotic lesions and inflammation |
| Cao et al., 2020 [50] | Cases: 24 Controls: 8 |
Sprague Dawley rats | Induced: autologous transplantation of uterine tissue fragments onto the peritoneal wall | Fecal pellets | NGS Illumina: V3-V4 16S rRNA | Aim: investigate if letrozole and SFZYD can act on microbiota, inhibiting the progression of lesions Conclusion: Letrozole and SFZYD reduce the inflammatory response in both ectopic and eutopic endometrial tissues, which could be associated with the decrease in the Firmicutes/Bacteroidetes ratio. |
| Ni et al., 2020 [51] | Cases: 16 Controls: 20 |
C57BL/6J mice | Induced: estrogen solution subcutaneous injection on days 1, 4, and 7, transplantation of endometrial fragments on day 8. Estrogen injection again on day 9, 11, and 14. After 3 weeks, mice were dissected to obtain faces from cecum. | Feces from cecum | NGS Illumina: V3-V4 16S rRNA | Aim: uncover the interaction between fecal metabolomics and gut microbiota in mice with endometriosis Conclusion: The abnormal fecal metabolites, particularly those related to secondary bile acid biosynthesis and the alpha-linolenic acid pathways, influenced by dysbiosis, may serve as distinctive features in mice with endometriosis and as potential markers for distinguishing the disease |
SFZYD, Shaofu Zhuyu decoction.
Table 2. Human clinical trials that explored the bidirectional relationship between gut microbiota and endometriosis.
| Study | N | Age (Years) | Endometriosis Diagnosis Type | Sample | Technic | Aim and Conclusion |
|---|---|---|---|---|---|---|
| Khan et al., 2016 [52] | Cases: 32 Controls: 32 In each group 16 in treatment with GnRHa |
21–52 | Surgery and histology | Endometrial swabs and cystic fluid | NGS Illumina: 16S rRNA | Aim: investigate microbial colonization in intrauterine environment and cystic fluid Conclusion: presence of sub-clinical infections in intrauterine environment and cystic fluid of ovarian endometriomas. Potential additional side effect of GnRHa treatment in promoting silent intrauterine and/or ovarian infections (abundance of Streptococcaceae, Staphylococaceae, Enterobacteriaceae and lowered Lactobacillae in GnRHa treated women) |
| Akiyama et al., 2019 [53] | Cases: 30 Controls: 39 |
20–44 | Surgery and histology | Cervical Mucus | NGS Illumina: V5-V6 16S rRNA | Aim: investigating pattern of microbiota in the cervical mucus Conclusion: Enterobacteriaceae and Streptococcus were more commonly found in women with endometriosis |
| Ata et al., 2019 [40] | Cases: 14 Controls: 14 |
18–45 | Surgery and histology (only stage 3–4) | Stool sample Vaginal and endocervical swabs |
NGS Illumina: V3- V4 16S rRNA | Aim: comparing gut, vaginal, and cervical microbiota in endometriosis vs. controls Conclusion: no differences in species level analysis, but they found a possible difference at genus level analysis |
| Chen et al., 2020 [54] | Cases: 20 endometriosis, 19 adenomyosis and 7 adenomyosis-endometriosis Controls: 36 |
18–45 | Surgery, histology, transvaginal ultrasound, and MRI | Cervical swabs and posterior fornix swabs | NGS Illumina: V3-V4 16S rRNA | Aim: create a microbiota profile model for endometriosis and investigate and identify significant microbiota associated with endometriosis or adenomyosis conditions. Conclusion: Higher prevalence of Atopobium in endometriosis-adenomysosis group |
| Hernandes et al., 2020 [42] | Cases: 10 Controls: 11 |
18–50 | Surgery and histology | Vaginal fluid, eutopic endometrium (collected by curettage), and endometrial lesion tissue samples (collected by surgery) | NGS Illumina: V3-V4 rRNA | Aim: Investigate and compare the microbiome profile Conclusion:
|
| Wei et al., 2020 [44] | Cases: 36 Controls: 14 |
23–44 | Surgery and histology | Lower reproductive tract swabs: lower third of the vagina, posterior vaginal fornix, cervical mucus Upper reproductive tract samples: surgery (endometrial and peritoneal lavage) |
NGS Ion Torrent PGM: V4-V5 16S rRNA | Aim: study the flora distribution and bacterial community across the upper and lower reproductive tract Conclusion: shift in microbiota distribution starting from the cervical samples (microbiota in cervical samples as an indicator for the risk of endometriosis) and progressively increasing upward the reproductive tract, decreased Lactobacillus in lower tract, enriched Sphingobium sp. and Pseudomonas viridiflava in endometrium and peritoneal fluid |
| Khan et al., 2021 [48] | Cases: 53 (21 untreated, 11 GnRHa, 15 LVFX, 6 LVFX+ GnRHa) Controls: 47 (11 untreated, 12 GnRHa, 10 LVFX, 14 LVFX+ GnRHa |
18–51 | Surgery and histology | Endometrial samples | NGS Illumina: V5-V6 16S rRNA Immunohistochimical analysis: Ab against CD138, CD68, Ki-67, and CD31 |
Aim: demonstrate the hypothesis that antibiotic treatment with or without GnRHa may decrease intrauterine infection with consequent decrease in tissue inflammation, cell proliferation and angiogenesis in human endometriosis Conclusion: Decreased Gardnerella, Prevotella, Acidibactor, Atopobium, Megasphaera, and Bradyrhizobium in patients with endometriosis in treatment with LVFX or LVFX + GnRHa, reduced occurrence rate of chronic endometritis after GnRHa + LVFX treatment comparison to GnRHa treatment group and decreased CD68, Ki-67, and CD31 |
LVFX, levofloxacin; GnRHa, gonadotropin-releasing hormone analogues.
This entry is adapted from the peer-reviewed paper 10.3390/ph16121696
References
- Giudice, L.C.; Kao, L.C. Endometriosis. Lancet 2004, 364, 1789–1799.
- Ahn, S.H.; Monsanto, S.P.; Miller, C.; Singh, S.S.; Thomas, R.; Tayade, C. Pathophysiology and Immune Dysfunction in Endometriosis. BioMed. Res. Int. 2015, 2015, 795976.
- Nisolle, M.; Donnez, J. Peritoneal endometriosis, ovarian endometriosis, and adenomyotic nodules of the rectovaginal septum are three different entities. Fertil. Steril. 1997, 68, 585–596.
- Santulli, P.; Tran, C.; Gayet, V.; Bourdon, M.; Maignien, C.; Marcellin, L.; Pocate-Cheriet, K.; Chapron, C.; de Ziegler, D. Oligo-anovulation is not a rarer feature in women with documented endometriosis. Fertil. Steril. 2018, 110, 941–948.
- Vercellini, P.; Parazzini, F.; Oldani, S.; Panazza, S.; Bramante, T.; Crosignani, P.G. Surgery: Adenomyosis at hysterectomy: A study on frequency distribution and patient characteristics. Hum. Reprod. 1995, 10, 1160–1162.
- Xholli, A.; Londero, A.P.; Mattos, L.C.; Vujosevic, S.; Cagnacci, A. Gynaecological pathologies leading to emergency department admissions: A cross-sectional study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2023, 282, 38–42.
- Nnoaham, K.E.; Hummelshoj, L.; Webster, P.; d’Hooghe, T.; de Cicco Nardone, F.; de Cicco Nardone, C.; Jenkinson, C.; Kennedy, S.H.; Zondervan, K.T. Impact of endometriosis on quality of life and work productivity: A multicenter study across ten countries. Fertil. Steril. 2011, 96, 366–373.e8.
- Tai, F.-W.; Chang, C.Y.-Y.; Chiang, J.-H.; Lin, W.-C.; Wan, L. Association of Pelvic Inflammatory Disease with Risk of Endometriosis: A Nationwide Cohort Study Involving 141,460 Individuals. J. Clin. Med. 2018, 7, 379.
- Domenis, R.; Pilutti, D.; Orsaria, M.; Marzinotto, S.; Candotti, V.; Bosisio, G.; Bulfoni, M.; Ruaro, M.E.; Di Loreto, C.; Della Mea, V.; et al. Expression and modulation of S100A4 protein by human mast cells. Cell. Immunol. 2018, 332, 85–93.
- Mariuzzi, L.; Domenis, R.; Orsaria, M.; Marzinotto, S.; Londero, A.P.; Bulfoni, M.; Candotti, V.; Zanello, A.; Ballico, M.; Mimmi, M.C.; et al. Functional expression of aryl hydrocarbon receptor on mast cells populating human endometriotic tissues. Lab. Investig. 2016, 96, 959–971.
- Jiang, I.; Yong, P.J.; Allaire, C.; Bedaiwy, M.A. Intricate Connections between the Microbiota and Endometriosis. Int. J. Mol. Sci. 2021, 22, 5644.
- DiVasta, A.D.; Vitonis, A.F.; Laufer, M.R.; Missmer, S.A. Spectrum of symptoms in women diagnosed with endometriosis during adolescence vs adulthood. Am. J. Obstet. Gynecol. 2018, 218, 324.e1–324.e11.
- Bloski, T.; Pierson, R. Endometriosis and chronic pelvic pain: Unraveling the mystery behind this complex condition. Nurs. Women’s Health 2008, 12, 382–395.
- Maroun, P.; Cooper, M.J.W.; Reid, G.D.; Keirse, M.J.N.C. Relevance of gastrointestinal symptoms in endometriosis. Aust. N. Z. J. Obstet. Gynaecol. 2009, 49, 411–414.
- Luscombe, G.M.; Markham, R.; Judio, M.; Grigoriu, A.; Fraser, I.S. Abdominal bloating: An under-recognized endometriosis symptom. J. Obstet. Gynaecol. Can. 2009, 31, 1159–1171.
- Svensson, A.; Brunkwall, L.; Roth, B.; Orho-Melander, M.; Ohlsson, B. Associations between endometriosis and gut microbiota. Reprod. Sci. 2021, 28, 2367–2377.
- Martin, D.H. The Microbiota of the Vagina and Its Influence on Women’s Health and Disease. Am. J. Med. Sci. 2012, 343, 2–9.
- Nelson, D.B.; Rockwell, L.C.; Prioleau, M.D.; Goetzl, L. The role of the bacterial microbiota on reproductive and pregnancy health. Anaerobe 2016, 42, 67–73.
- Qin, R.; Tian, G.; Liu, J.; Cao, L. The gut microbiota and endometriosis: From pathogenesis to diagnosis and treatment. Front. Cell. Infect. Microbiol. 2022, 12, 1069557.
- Sinaii, N.; Plumb, K.; Cotton, L.; Lambert, A.; Kennedy, S.; Zondervan, K.; Stratton, P. Differences in characteristics among 1000 women with endometriosis based on extent of disease. Fertil. Steril. 2008, 89, 538–545.
- Laschke, M.W.; Menger, M.D. The gut microbiota: A puppet master in the pathogenesis of endometriosis? Am. J. Obstet. Gynecol. 2016, 215, 68.e1–68.e4.
- Zhu, S.; Jiang, Y.; Xu, K.; Cui, M.; Ye, W.; Zhao, G.; Jin, L.; Chen, X. The progress of gut microbiome research related to brain disorders. J. Neuroinflamm. 2020, 17, 25.
- Talwar, C.; Singh, V.; Kommagani, R. The gut microbiota: A double-edged sword in endometriosis. Biol. Reprod. 2022, 107, 881–901.
- Possemiers, S.; Bolca, S.; Verstraete, W.; Heyerick, A. The intestinal microbiome: A separate organ inside the body with the metabolic potential to influence the bioactivity of botanicals. Fitoterapia 2011, 82, 53–66.
- Elkafas, H.; Walls, M.; Al-Hendy, A.; Ismail, N. Gut and genital tract microbiomes: Dysbiosis and link to gynecological disorders. Front. Cell. Infect. Microbiol. 2022, 12, 1059825.
- Wei, Y.; Tan, H.; Yang, R.; Yang, F.; Liu, D.; Huang, B.; OuYang, L.; Lei, S.; Wang, Z.; Jiang, S.; et al. Gut dysbiosis-derived β-glucuronidase promotes the development of endometriosis. Fertil. Steril. 2023, 120, 682–694.
- Pai, A.H.-Y.; Wang, Y.-W.; Lu, P.-C.; Wu, H.-M.; Xu, J.-L.; Huang, H.-Y. Gut Microbiome–Estrobolome Profile in Reproductive-Age Women with Endometriosis. Int. J. Mol. Sci. 2023, 24, 16301.
- Troha, N.; Rižner, T.L. Medsebojni vpliv estrogenov in mikrobioma prebavil in rodil. Slov. Med. J. 2023, 92, 335–344.
- Baker, J.M.; Al-Nakkash, L.; Herbst-Kralovetz, M.M. Estrogen–gut microbiome axis: Physiological and clinical implications. Maturitas 2017, 103, 45–53.
- E Salliss, M.; Farland, L.V.; Mahnert, N.D.; Herbst-Kralovetz, M.M. The role of gut and genital microbiota and the estrobolome in endometriosis, infertility and chronic pelvic pain. Hum. Reprod. Updat. 2021, 28, 92–131.
- Beaud, D.; Tailliez, P.; Anba-Mondoloni, J. Genetic characterization of the β-glucuronidase enzyme from a human intestinal bacterium, Ruminococcus gnavus. Microbiology 2005, 151, 2323–2330.
- Leonardi, M.; Hicks, C.; El-Assaad, F.; El-Omar, E.; Condous, G. Endometriosis and the microbiome: A systematic review. BJOG Int. J. Obstet. Gynaecol. 2019, 127, 239–249.
- Qi, X.; Yun, C.; Pang, Y.; Qiao, J. The impact of the gut microbiota on the reproductive and metabolic endocrine system. Gut Microbes 2021, 13, 1894070.
- Hu, S.; Ding, Q.; Zhang, W.; Kang, M.; Ma, J.; Zhao, L. Gut microbial beta-glucuronidase: A vital regulator in female estrogen metabolism. Gut Microbes 2023, 15, 2236749.
- Bailey, M.T.; Coe, C.L. Endometriosis is associated with an altered profile of intestinal microflora in female rhesus monkeys. Hum. Reprod. 2002, 17, 1704–1708.
- Sobstyl, A.; Chałupnik, A.; Mertowska, P.; Grywalska, E. How Do Microorganisms Influence the Development of Endometriosis? Participation of Genital, Intestinal and Oral Microbiota in Metabolic Regulation and Immunopathogenesis of Endometriosis. Int. J. Mol. Sci. 2023, 24, 10920.
- Huang, L.; Liu, B.; Liu, Z.; Feng, W.; Liu, M.; Wang, Y.; Peng, D.; Fu, X.; Zhu, H.; Cui, Z.; et al. Gut microbiota exceeds cervical microbiota for early diagnosis of endometriosis. Front. Cell. Infect. Microbiol. 2021, 11, 788836.
- Sakamoto, Y.; Harada, T.; Horie, S.; Iba, Y.; Taniguchi, F.; Yoshida, S.; Iwabe, T.; Terakawa, N. Tumor necrosis factor-α-induced interleukin-8 (IL-8) expression in endometriotic stromal cells, probably through nuclear factor-κB activation: Gonadotropin-releasing hormone agonist treatment reduced IL-8 expression. J. Clin. Endocrinol. Metab. 2003, 88, 730–735.
- Khan, K.N.; Fujishita, A.; Kitajima, M.; Hiraki, K.; Nakashima, M.; Masuzaki, H. Intra-uterine microbial colonization and occurrence of endometritis in women with endometriosis. Hum. Reprod. 2014, 29, 2446–2456.
- Ata, B.; Yildiz, S.; Turkgeldi, E.; Brocal, V.P.; Dinleyici, E.C.; Moya, A.; Urman, B. The endobiota study: Comparison of vaginal, cervical and gut microbiota between women with stage 3/4 endometriosis and healthy controls. Sci. Rep. 2019, 9, 2204.
- Chadchan, S.B.; Cheng, M.; A Parnell, L.; Yin, Y.; Schriefer, A.; Mysorekar, I.U.; Kommagani, R. Antibiotic therapy with metronidazole reduces endometriosis disease progression in mice: A potential role for gut microbiota. Hum. Reprod. 2019, 34, 1106–1116.
- Hernandes, C.; Silveira, P.; Rodrigues Sereia, A.F.; Christoff, A.P.; Mendes, H.; Valter de Oliveira, L.F.; Podgaec, S. Microbiome profile of deep endometriosis patients: Comparison of vaginal fluid, endometrium and lesion. Diagnostics 2020, 10, 163.
- Lee, S.-R.; Lee, J.-C.; Kim, S.-H.; Oh, Y.-S.; Chae, H.-D.; Seo, H.; Kang, C.-S.; Shin, T.-S. Altered Composition of Microbiota in Women with Ovarian Endometrioma: Microbiome Analyses of Extracellular Vesicles in the Peritoneal Fluid. Int. J. Mol. Sci. 2021, 22, 4608.
- Wei, W.; Zhang, X.; Tang, H.; Zeng, L.; Wu, R. Microbiota composition and distribution along the female reproductive tract of women with endometriosis. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 15.
- Yuan, M.; Li, D.; Zhang, Z.; Sun, H.; An, M.; Wang, G. Endometriosis induces gut microbiota alterations in mice. Hum. Reprod. 2018, 33, 607–616.
- Liu, Z.; Chen, P.; Luo, L.; Liu, Q.; Shi, H.; Yang, X. Causal effects of gut microbiome on endometriosis: A two-sample mendelian randomization study. BMC Women’s Health 2023, 23, 637.
- Uzuner, C.; Mak, J.; El-Assaad, F.; Condous, G. The bidirectional relationship between endometriosis and microbiome. Front. Endocrinol. 2023, 14, 1110824.
- Khan, K.N.; Fujishita, A.; Muto, H.; Masumoto, H.; Ogawa, K.; Koshiba, A.; Mori, T.; Itoh, K.; Teramukai, S.; Matsuda, K.; et al. Levofloxacin or gonadotropin releasing hormone agonist treatment decreases intrauterine microbial colonization in human endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 264, 103–116.
- Kalinkina, O.B.; Tezikov, Y.V.; Lipatov, I.S.; Aravina, O.R.; Morozova, O.N. Study of urogenital tract and gut microbiota in women with stage III and IV ovarian endometriosis. Perm Med. J. 2020, 37, 14–21.
- Cao, Y.; Jiang, C.; Jia, Y.; Xu, D.; Yu, Y. Letrozole and the Traditional Chinese Medicine, Shaofu Zhuyu Decoction, Reduce Endometriotic Disease Progression in Rats: A Potential Role for Gut Microbiota. Evid.-Based Complement. Altern. Med. 2020, 2020, 3687498.
- Ni, Z.; Sun, S.; Bi, Y.; Ding, J.; Cheng, W.; Yu, J.; Zhou, L.; Li, M.; Yu, C. Correlation of fecal metabolomics and gut microbiota in mice with endometriosis. Am. J. Reprod. Immunol. 2020, 84, e13307.
- Khan, K.N.; Fujishita, A.; Masumoto, H.; Muto, H.; Kitajima, M.; Masuzaki, H.; Kitawaki, J. Molecular detection of intrauterine microbial colonization in women with endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 199, 69–75.
- Akiyama, K.; Nishioka, K.; Khan, K.N.; Tanaka, Y.; Mori, T.; Nakaya, T.; Kitawaki, J. Molecular detection of microbial colonization in cervical mucus of women with and without endometriosis. Am. J. Reprod. Immunol. 2019, 82, e13147.
- Chen, S.; Gu, Z.; Zhang, W.; Jia, S.; Wu, Y.; Zheng, P.; Dai, Y.; Leng, J. Microbiome of the lower genital tract in Chinese women with endometriosis by 16s-rRNA sequencing technique: A pilot study. Ann. Transl. Med. 2020, 8, 1440.
This entry is offline, you can click here to edit this entry!
