The biological history of Chlamydia trachomatis is intertwined with the evolution of the man. Infecting Elemental Bodies (EBs), having penetrated mucosal epithelial cells, wrap themselves in a cloak (ĸλαμις) of glycogen that ensures their obligatory intracellular survival and protects this differentiation into Reticulate Bodies (RBs) that feed on cellular ATP. Multiple chemokines and cytokines are involved under the direction of IL-6 in the florid phase and IL-17A in the scar phase. The WHO has successfully identified the SAFE strategy against trachoma (Surgery, Antibiotics, Facial cleansing, Environment) as the blueprint to eliminate the disease by 2020. Recently, interest has been increasingly focused on changing sexual attitudes in different areas of the world, leaving Musca sorbens, Scatophaga stercoraria, and stepsisters fairly blameless, but extolling the role of Chlamydia trachomatis in apparently “sterile” chronic prostatitis or conjunctivitis or, less frequently, in oropharyngitis and proctitis. The addition of an S (SAFE-S) standing for “sexual behavior” was then proposed to also attract the interest and attention not only of Ophthalmologists and Obstetricians/Gynecologists, Urologists/Andrologists, and the School Authorities for information on the prevention of sexually transmitted diseases, but also of Social Physicians and Pediatricians. This means that sexually transmitted infections should be screened in asymptomatic patients with risky sexual behavior or sexual contact with people diagnosed with a transmitted infection.
- trachoma
- chlamydia
- WHO SAFE strategy
- RT-PCR
- conjunctivitis
- sexually transmitted diseases
- neglected transmitted diseases
- Musca sorbens
- vaccines
1. History and Italian Background

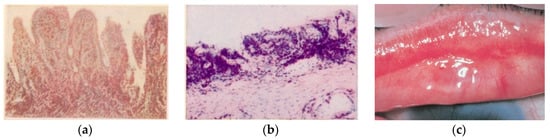
2. Contagion and Clinic, The SAFE-S Strategy


Having this scenario clear, the researchers proposed to complete the acronym SAFE with -S (SAFE-S), understood as ''strategy for controlling sexual well-being, i.e.: sexual behaviour'' [20]. Unprotected sex is guilty: the WHO recomendations for the next decade 2021-2030 must attract the interest and attention not only of Obstetricians/Gynecologists, Urologists/Andrologists and Pediatricians, but also High School Authorities that should be mandated to improve students' knowledge on sexually transmitted disease education and prophylaxis/protection.
3. Conclusions
The oculo-genital correlation and the need for adequate systemic coverage are confirmed by cases of chronic conjunctival disease resolved with ultrasound-guided infiltrative therapy of chronic prostatitis [31] or recurrent atypical chronic oropharyngeal inflammation resolved after chlamydial antibiotic therapy [32].
Therefore, the researchers consider the occasion of this edition useful to relaunch an alert also for the Practitioners and the Social Physicians to holistically evaluate the patient, and in front of multisite infection “check for the presence of typical signs (Arlt’s line [5]; Neri’s white line [33]) or consequences: entropion and pannus, infertility [34], spontaneous abortion, pelvic inflammatory disease” [1][35], cervical cancer in association with HPV, and proctitis especially in homosexual males [36]. Ct also appears to be involved in oculo-orbital lymphoma [37], previously associated only with Chlamydia psittaci. New research would be desirable to confirm or exclude the hypothesis of Chlamydia pneumoniae as a chronic inflammatory stimulus for neovascular Age-related Macular Degeneration (nAMD) [38], inducing the inflammatory state [39]. Thus, it seems appropriate to draw the attention to Chlamydia for an evaluation of florid or chronic conjunctivitis, as well as for oropharyngeal diseases, such as Neisseria gonorrhoeae and other sexually transmitted diseases [40] which could also trigger, sometimes, reactive arthritis [41][42]. The PAHO-WHO plans to control the worldwide trachoma infection by 2020, between 2018 and 2019 showed reduction of 14,4 million people that lived in areas with high trachoma prevalence, but still leaving a total burden of 2,5 million cases of trachomatous trichiasis in 2019. This urgently requires an increase in medical and surgical strategy with decisive support for research and development of an effective and durable vaccine against chlamydial and mycoplasmal infections [43][44], improving the prevention by considering SAFE-S strategy, rather than insect powder.
This entry is adapted from the peer-reviewed paper 10.3390/pathogens12121419
References
- Oriel, J.D.; Ridgway, G.L. Genital Infection by Chlamydia Trachomatis; Elsevier Biomedical USA: Gainesville, FL, USA, 1982.
- Mackenzie, W. Traité Pratique des Maladies De l’Oeil; In Trad Franç, 4th ed.; Masson: Paris, France, 1856.
- Gallenga, P.E. Storia della cataratta nel secondo dopoguerra. In Evoluzione Della Chirurgia Della Cataratta in Italia; Storia, Buratto, L., Eds.; Fabiano: Moasca, Italy, 2019; pp. 1–40.
- Gallenga, C. Sul trattamento operativo delle cicatrici corneali. Gazz. Clin. 1885, 21, 1–9.
- Gallenga, C. Seconda osservazione di concrezione calcarea delle palpebre. Gazz. Clin. 1886, 3, 1–2.
- von Prowazek, S.; Halberstädter, L. Zur Aetiologie des Trachoms. Dtsch. Med. Wochenschr. 1907, 33, 1285–1287.
- Gallenga, C. Della Specificità Dei Corpuscoli Di Prowazeck E Halberstaedter Nella Congiuntivite Granulomatosa; Atti Società Italiana Patologia, VI riunione; Società Tipografica Modenese; Antica Tipografia Soliani: Modena, Italy, 1909.
- Gallenga, C. Zur Färbung der Prowazeck Halberstädterischen Trachom-Körperchen. Klin. Augenheilkd. 1910, 1, 195s.
- Axenfeld, T. Traité d’Ophtalmologie; Steinheil: Paris, France, 1914.
- Dark, A.J. Inclusion bodies in trachoma. Brit. J. Ophth. 1955, 39, 751.
- Gallenga, C.E.; Del Boccio, M.; Del Boccio, G.; Perri, P.; Contini, C. Who is afraid of a bug mantled like Zorro? EC Microbiol. 2019, 15, 511–513. Available online: www.researchgate.net/publication/334251061 (accessed on 20 January 2023).
- Bietti, A. Trattato di Oftalmoiatria; Istituto Editoriale Scientifico: Milan, Italy, 1925.
- Gallenga, R. Plasmoma Della Congiuntiva Bulbare Superiore; Atti Congr Soc It Oftalmol: Roma, Italy, 1931; L’Universale Tipografia Poliglotta: Roma, Italy, 1932.
- Gallenga, R. The Ophthalmology course, duplicated lecture notes (Gagna E. typographer. 5th year Medical School University of Turin). 1966; and OMC&O Turin conference, in the University Eye Clinic 1967.
- Scuderi, G. Istopatologia Del Trachoma; Minerva Medica: Turin, Italy, 1957.
- Contini, A. Un Sassarese in Un Tempio Della Scienza; EDES: Sassari, Italy; ISBN 9788860252081.
- Frezzotti, R.; Guerra, R. Oftalmologia Essenziale, 2nd ed.; CEA: Milano, Italy, 2006.
- Schachter, J.; Causse, G.; Tarizzo, M.L. Chlamydiae as agents of sexually transmitted disease. WHO Bull. 1976, 54, 245–254.
- Delle Noci, N. George Bartisch. L’Ophthalmodouleia e l’Oculistica Rinascimentale; Théa special ed; Centro Grafico srl: Foggia, Italy, 2019.
- Gallenga, P.E.; Del Boccio, M.; Gallenga, C.E.; Neri, G.; Pennelli, A.; Toniato, E.; Lobefalo, L.; Maritati, M.; Perri, P.; Contini, C. Diagnosis of a neonatal ophthalmic discharge, Ophtalmia Neonatorum, in the ‘Molecular age’: Investigation for a correct therapy. J. Biol. Regul. Homeost. Agents 2018, 32, 177–184.
- Akinsolu, F.T.; Nemieboka, P.O.; Njuguna, D.W.; Ahadji, M.N.; Dezso, D.; Varga, O. Emerging resistance of neglected tropical diseases: A scoping review of the literature. Int. J. Environ. Res. Public Health. 2019, 16, 1925.
- Lewies, A.; Wentzel, J.F.; Jacobs, G.; Du Plessis, L.H. The potential use of natural and structural Antimicrobial Peptides in the fight against neglected Tropical Diseases. Molecules 2015, 20, 15392–15433.
- Quin, J. Gene and peptide in the treatment of chlamydia trachomatis infections: Beginning of an era? Theoretical. Nat. Sci. 2023, 4, 18–25.
- Astrauskiene, D.; Griskevicius, A.; Luksiene, R.; Panaviene, V.; Venaliene, J. Chlamydia trachomatis, Ureaplasma urealyticum, and Mycoplasma hominis in sexually intact girls with arthritides. Scand. J. Rheumatol. 2012, 41, 275–279.
- Bailey, R.; Lietman, T. The SAFE strategy for the elimination of trachoma by 2020: Will work? Bull. World Health Organ. 2001, 79, 233–236.
- WHO. Alliance for the Global Elimination of Trachoma by 2020: Progress report, 2019. WER 2020, 30, 349–360.
- WHO. Ending the Neglect to Attain the Sustainable Development Goals: A Rationale for Continued Investment in Tackling Neglected Tropical Diseases 2021–2030; WHO: Geneva, Switzerland, 2022; ISBN 9789240052932.
- Robinson, A.; Bristow, J.; Holl, M.V.; Makalo, P.; Alemayehu, W.; Bailey, R.L.; Macleod, D.; Birkett, M.A.; Caulfield, J.C.; Sarah, V. Responses of the putative trachoma vector, Musca sorbens, to volatile semiochemicals from human faeces. PLoS Negl. Trop. Dis. 2020, 14, e0007719.
- Hirschberger, P.; Degro, H.N. Oviposition of the dung beetle Aphodius ater in relation to the abundance of yellow dung fly larvae (Scatophaga stercoraria). Ecol. Entomol. 1996, 21, 352–357.
- Blanckenhorn, W.U.; Pemberton, A.J.; Bussière, L.F.; Roembke, J.; Floate, K.D. A Review of the Natural History and Laboratory Culture Methods for the Yellow Dung Fly, Scathophaga stercoraria. J. Insect Sci. 2010, 10, 11.
- Gallenga, P.E.; Del Boccio, M.; Neri, G.; Tenaglia, R.; Martinotti, S. Conjunctival chronic pain by Chlamydia trachomatis solved after asymptomatic chronic prostatitis treatment with echoguided drugs infiltration: A case report. Int. J. Med. Clin. Res. 2011, 2, 93–97.
- Neri, G.; Del Boccio, M.; Pennelli, A.; Martinotti, S.; Tenaglia, R.; Pugliese, M.; Toniato, E.; Croce, A.; Gallenga, P.E. Jugulodigastric lymph node inflammation derived from chronic atypical oropharyngeal phlogosis recurring annually after flu virus vaccination: A holistic vision of a clinical case solved after chlamydicidal antibiotic therapy. Int. J. Immunopathol. Pharmacol. 2012, 25, 835–847.
- Neri, G.; Pugliese, M.; Castriotta, A.; Mastronardi, V.; Pasqualini, P.; Colasante, A.; Cazzato, F.; Talamonti, R.; Del Boccio, G. White-line: A new finding in laryngopharyngeal reflux objective evaluation. Med. Hypotheses 2013, 80, 769–772.
- Di Bonaventura, G.; Del Boccio, M.; Pennelli, A.; Rapinese, M.; Tenaglia, R.; Gallenga, P.E.; Neri, G.; Boccio, G.D. Unusual Oropharyngeal Asymptomatic Manifestations Caused by Atypical Pathogens Detected by PCR into Altered Ecosystems of an Infertile Couple. Microbiol. Res. J. Int. 2014, 5, 447–458.
- Neri, G.; Pennelli, A.; Del Boccio, M.; Neri, L.; Gallenga, C.E.; Toniato, E.; Tenaglia, R. Molecular Evidences of Chlamydia Trachomatis and Urogenital Mycoplasmas Infections from Birth Evolving into Multisite Infections. EC Microbiol. 2019, 15, 385–394. Available online: https://ecronicon.net/assets/ecmi/pdf/ECMI-15-00639.pdf (accessed on 20 January 2023).
- Mei, C. Confronto di Metodiche Analitiche, Immunocromatografiche e Biomolecolari per la Determinazione di Chlamydia Trachomatis in Campioni Biologici. Ph.D. Thesis, Università C. Bo, Urbino, Italy, 2017.
- Contini, C.; Seraceni, S.; Carradori, S.; Cultrera, R.; Perri, P.; Lanza, F. Identification of Chlamydia trachomatis in a patient with ocular lymphoma. Am. J. Hematol. 2009, 84, 597–599.
- Robman, L.; Mahdi, O.; McCarty, C.; Dimitrov, P.; Tikellis, G.; McNeil, J.; Byrne, G.; Taylor, H.; Guymer, R. Exposure to Chlamydia pneumoniae infection and progression of age-related macular degeneration. Am. J. Epidemiol. 2005, 161, 1013–1019.
- Gallenga, C.E.; Parmeggiani, F.; Costagliola, C.; Sebastiani, A.; Gallenga, P.E. Inflammaging: Should this term be suitable for age related macular degeneration too? Inflamm. Res. 2014, 63, 105–107.
- Breyer, B.N.; Huang, W.H.; Rabkin, C.S.; Alderete, J.F.; Pakpahan, R.; Beason, T.S.; Kenfield, S.A.; Mabie, J.; Ragard, L.; Wolin, K.Y.; et al. Sexually transmitted infections, benign prostatic hyperplasia and lower urinary tract symptom-related outcomes: Results from the Prostate, Lung, Colorectal and Ovarian Screening Trial. Brit. J. Urol. 2016, 117, 145–154.
- Mabey, D.C.; Solomon, A.W.; Foster, A. Trachoma. Lancet 2003, 362, 223–229.
- Wright, H.R.; Turner, A.; Taylor, H.R. Trachoma. Lancet 2008, 371, 1945–1954.
- Stary, G.; Olive, A.; Radovic-Moreno, A.F.; Gondek, D.; Alvarez, D.; Basto, P.A.; Perro, M.; Vrbanac, V.D.; Tager, A.M.; Shi, J.; et al. Vaccines. A mucosal vaccine against Chlamydia trachomatis generates two waves of protective memory T cells. Science 2015, 348, 8205.
- Gottlieb, S.L.; Johnston, C. Future prospects for new vaccines against sexually transmitted infections. Curr. Opin. Infect Dis. 2017, 30, 77–86.
